 Emeritus Professor
Emeritus Professor
216.368.3712 rcd@case.edu Millis 225D
Interests: Chemical Biology, Biophysical Chemistry, Organometallic Chemistry, Photochemistry, Physical Chemistry, Astrochemistry, Chemical Kinetics, Computational Chemistry, Ion Chemistry, Spectroscopy, Theoretical Chemistry, Thermochemistry
BA, Harvard University, 1965
PhD, Stanford University, 1970
Alfred P. Sloan Fellow, 1973-75
CWRU Sigma Xi Research Award, 1977
J.S. Guggenheim Fellow, 1978-79
 Dunbar Curriculum Vitae (pdf)
Dunbar Curriculum Vitae (pdf)
Fourier Transform Mass Spectrometry and Ion Cyclotron Resonance Spectrometry
Basic tools used in Dr. Dunbar’s research are mass spectometers, in particular FTMS and ICR instrumentation. The group is a world leader in developing the possibilities of combining lasers with ion-trapping mass spectrometers. Experiments are collaborative with groups in the Netherlands and Florida.
Binding of Metal Ions
The binding of metal ions to ligands is explored in the solvent-free gas-phase environment of the mass spectrometer cell. Probing binding sites of metal ions with amino acids as models gives insight into their interaction with protein surfaces in biological systems. Interaction of ions with both flat and curved carbon surfaces modeled on graphite sheds light on electrochemistry and catalysis.
Interstellar and Circumstellar Chemistry
We have worked out some powerful new theoretical tools in conjunction with our experimental work with metal ion chemistry in laboratory vacuum environments. These theoretical tools have turned out to be useful in understanding the deep-space chemistry occurring in interstellar clouds, and also in the “dense” circumstellar envelopes surrounding some remarkable carbon-rich stars. Exotic molecules like magnesium cyanotriacetylene ion turn out to be important there.
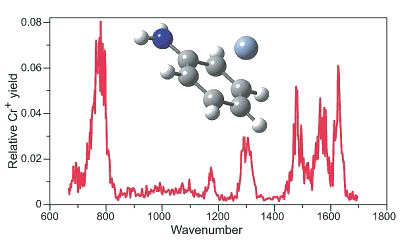
Ion Spectroscopy with the Free Electron Laser
In collaboration with the free electron laser facility in Nieuwegein, Netherlands, we are developing remarkable new approaches to spectroscopy combining the laser with the mass spectrometer. Infrared spectra of some of our metal-ion complexes (such as derivatives of dibenzene chromium ion) are among the most exciting new developments in the study of the structures and properties of such gas-phase ions.
Computational Chemistry
Modern quantum chemistry does chemical “experiments” in the computer by solving the Schrodinger equation of the reacting system. We are using these powerful tools to carry out metal-ion binding studies in parallel with the experimental work. We use the resources of the Ohio Supercomputer Center, but also find our desktop PC’s to be almost equally powerful in this work.
Selected Publications
- M. Vala, J. Szczepanski, R. C. Dunbar, J. Oomens and J. D. Steill, “Infrared Multiphoton Dissociation Spectrum of Isolated Protonated 1-Azapyrene,” Chem. Phys. Lett., 473, 43-48 (2009).
- R. C. Dunbar, J. D. Steill, and J. Oomens, “Chirality-Induced Conformational Preferences in Peptide Metal-Ion Binding Revealed by IR Spectroscopy,” J. Am. Chem. Soc., 133, 1212-15 (2011).
- T. Baer and R. C. Dunbar, “Ion Spectroscopy: Where did it come from; where is it now; and where is it going?” J. Am. Soc. Mass Spectrom., 21, 681-693 (2010).
- J. Green and R. C. Dunbar, J. “When do molecular bowls encapsulate metal cations?” J. Phys. Chem. A, 115, 4968-75 (2011).
- R. C. Dunbar, J. D. Steill, and J. Oomens, “Encapsulation of Metal Cations by the PhePhe Ligand,” J. Am. Chem. Soc., 133, 9376-86 (2011).
