Overview
Our research aims at developing and studying novel hybrid organic/inorganic materials with interesting optoelectronic properties such as intense light absorption and good charge transport properties. These types of materials lead to interesting fundamental studies as well as enable a variety of applications, including solar cells and transistors. Our group has traditionally done both synthesis of new compounds, and physical characterization in solution, film and devices. Series of compounds are designed to test hypotheses and obtain structure/property relationships. Our group works as a team, and group members are encouraged to collaborate with others to solve problems.
Keywords: Physical, organic, inorganic, polymer and materials chemistry; hybrid organic/inorganic materials, dyes, charge transport, solar energy conversion, organic electronics.
1) Electron acceptors for Solution-processable Organic Photovoltaics (OPV)
Solution-processable organic solar cells have the potential to become a disruptive solar technology — “solar paint”– that is inexpensive, efficient, and mass produced. Indeed, considerable research over the past 15 years has led to power conversion efficiencies of nearly 12% for single junction devices. Until 2012, most of the development of new active materials was focused on optimizing the electron donor that was combined with fullerene-based acceptors. Much less work had been devoted to the discovery and study of new electron acceptors. However, to push the envelop further, we need to go beyond fullerene, because fullerenes have disadvantages such as poor visible to near IR light absorption and poor tuning of the energy levels. Our research aims at creating and studying novel alternative non-fullerene acceptors to enable the next generation of OPV and other optoelectronic devices.
 The efficiency of OPV depends on many parameters that must be optimized simultaneously, making the development of new successfull materials very difficult. In addition to absorbing visible to near-IR light and have tunable energy levels, the acceptor must have high charge carrier mobility and form a favorable morphology when blended with the electron donor. This requires getting the right balance between self-aggregation and miscibility.
The efficiency of OPV depends on many parameters that must be optimized simultaneously, making the development of new successfull materials very difficult. In addition to absorbing visible to near-IR light and have tunable energy levels, the acceptor must have high charge carrier mobility and form a favorable morphology when blended with the electron donor. This requires getting the right balance between self-aggregation and miscibility.
The highest efficiency we could get using naphthalene diimides as acceptor blended with P3HT as donor was about ~1% We believe that it is due to its small planar shape that makes it hard to make good electron paths, especially in mixed donor/acceptor phases.
In our NSF CAREER funded efforts, we discovered that azadipyrromethene-based Zn(II) complexes (Zn(WS3)2) are strong electron acceptors. These complexes are unique in that they are large, non-planar and conjugated over the entire molecule due to π-conjugation within the ligands and interligand π- π interactions. OPVs using Zn(WS3)2 acceptor showed a power conversion efficiency as high as 4% and a charge carrier mobility of 10-4 cm2/Vs (Space Charge Limiting Current (SCLC)), impressive for a solution-processed non-planar transition metal complex. The Zn(II) metal center does not participate in the frontier molecular orbitals, so the properties are governed mostly by the ligands. Conjugated ‘arms’ projects from the core in several directions, thus enabling isotropic charge transport in films. Isotropic charge transport is desirable in diode-type devices such as OPVs and OLEDs, but also in other electronic applications such as OFETs because it allows for making films with the same electrical properties regardless of orientation or processing, a useful property for enabling commercialization. We are now doing structure-property studies to further tune their properties and provide fundamental understanding of charge transport in this new type of semiconductors.
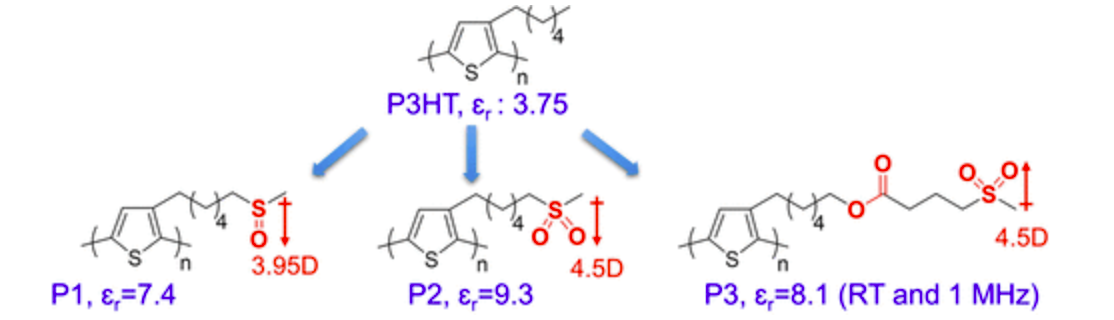
2) Organic semiconductors with high dielectric constant
We have an active collaboration with Prof. Lei Zhu and Prof. Morton Litt in the Macromolecular Science and Engineering department at CWRU to develop and study semiconducting polymers with high dielectric constant. Compared to inorganic semiconductors, organic semiconductors have the advantage of good mechanical properties that enables flexible devices, but typically have low relative dielectric constant (εr), which means that in a solar cell, one needs to use both a donor and an acceptor material with off-set energy levels to split the exciton, thus lowering maximum open-circuit voltage (VOC) and power conversion efficiency (PCE). The low dielectric constant also leads to more charge recombination. Here, we are designing new organic materials with high dielectric constant by increasing either their dipolar or electronic polarization. We then synthesize novel materials and test whether these new materials will have enhanced charge transport in films and whether they can break current fundamental limitations of excitonic solar cells and enable organic solar cells with 20% PCEs.
Techniques used by our group members
Synthesis of molecules and polymers, Schlenk-line technique, NMR, GC/MS, GPC, MALDI-TOF MS, TGA, DSC, UV/Vis absorption spectroscopy, fluorescence spectroscopy, cyclic voltammetry, infrared spectroscopy, thin-film formation, AFM, TEM, fabrication of organic solar cells, measurement of solar cell performance, DFT calculations, multidisciplinary research.
Video:Perspective on designing alternative molecular electron acceptors for OPVs