| |
|
|
|
|
Driving a stimulus current into a tissue medium requires
charging the electrode/electrolyte interface and charge transfer
across this electrode/electrolyte interface. In the case
of cathodic stimulation, electrons move from the electrode
to the electrolyte and in the case of anodic stimulation,
electrons move from the electrolyte to the electrode. For
an electron, at a given energy level, to move across the
interface an unoccupied state at the same energy level must
exist for radiationless transfer. For neural stimulating
electrodes that are at rest (not pulsed), no transfer of
electrons occurs because all electron energy states are occupied
on both sides of the interface. Charging or discharging the
metal electrode will align occupied, donor electrons, and
unoccupied energy states, receiving or acceptor states, for
charge transfer to occur. In the following sections, the
concept of moving electrons across the interface by charging
and discharging the electrode will be presented. The charge
transfer process presented here draws from work in the 1950s
and 60s credited to Rudolph A. Marcus, Nobel Prize, 1992.
(A much more detailed account of this can be found in Electrochemical Methods
Fundamentals and Applications, A.J. Bard and L.R. Faulkner, 2nd edition, John
Wiley & Sons, Inc.)
|
 The ‘free electron’ model
of metals assumes that the conduction electrons of the
valence band are not
confined
to individual atoms. They were distributed through the
metal like an electron gas. Interactions of the electrons
with
the core metal ions and with each other are neglected. The ‘free electron’ model
of metals assumes that the conduction electrons of the
valence band are not
confined
to individual atoms. They were distributed through the
metal like an electron gas. Interactions of the electrons
with
the core metal ions and with each other are neglected.
From quantum theory, we know that electrons must have different
energy states. Electrons having half-integer magnetic spins
are Fermions and their energy levels are described by the
Fermi-Dirac distribution.
F(E) = 1/[{exp(E – Ef/kT}
+ 1], where Ef is a parameter called the Fermi level.
At E = Ef, F(E) = 1/2, so at the Fermi energy
level the probability of electron occupation is 1/2.
At T=0, F(E) =1 for E < Ef and F(E) = 0 for
E > Ef,
so the probability of occupation is a step function at
Ef . So, at a temperature of absolute
zero, all available energy
states up to EF are fully occupied and all states above
are empty. This situation is generally considered approximately
true even at room temperature.
The number of electron states between E and E+dE is given
by Az(E)dE. Where A is the area of electrode in
contact with the electrolyte and z(E) is the density
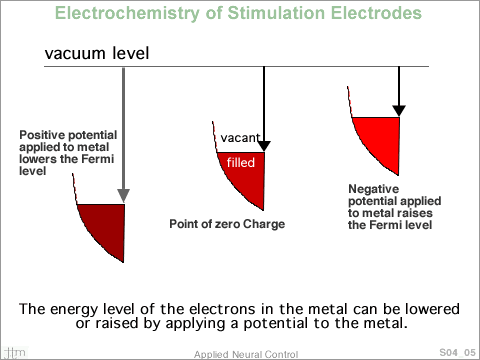
of electron states. In the figure, the boundary outlines
the allowable electron
energy density of states in a hypothetical metal. At T =
0, all available energy levels below Ef are filled
and all energy levels above Ef are vacant.
The Fermi level of the metal can be raised or lowered by
an applied electrical potential. When charge is added to
or subtracted from the electrode all electron states are
shifted upward or downward along the energy axis.
|
|
|
|
|
|
|
|

