2024 READI Themed Pilot Awardees
Ten academic-community research teams will embark on a year-long journey to increase diversity in clinical trials by eliminating participation barriers through community engagement.
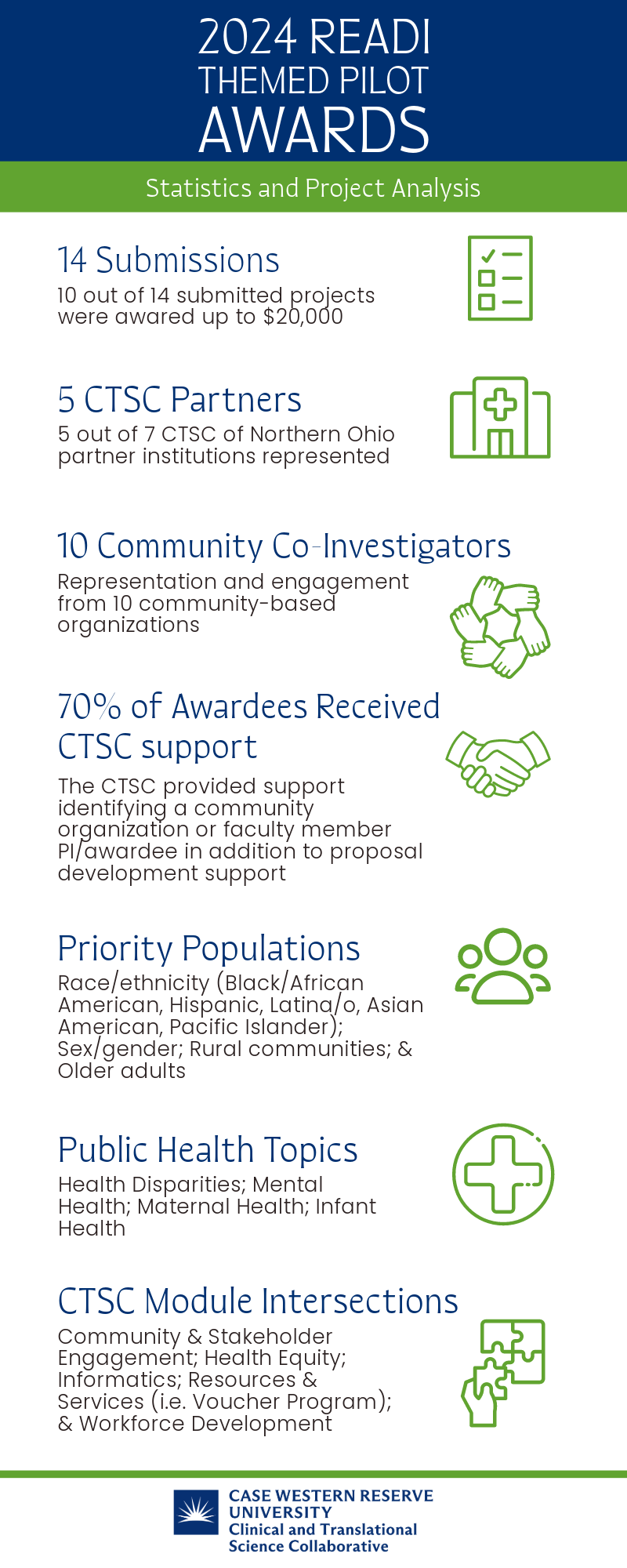
April 1, 2024 -- Congratulations to our 2024 READI Themed Pilot Awardees! Requests were submitted for pilot research funding to increase diversity in clinical trials and enhance community engagement by addressing barriers to clinical trial participation. Ten pilot grants with awards up to $20,000 were presented to the following institutions and community organizations.
1. Promoting Research Equity by Diversifying Clinical Trial Enrollment: A Vision of Change Community Health Worker CTSC Pilot
Team:
- PI: Kurt Stange, MD (CWRU)
- Community Co-I: Delores Collins, Executive Director, A Vision of Change
- Study Team: Robin Gotler, MA (CWRU), Michael Matthews, MA (A Vision of Change), Samia Marchmon, C-CHEW (A Vision of Change), Janterria Matthews, DNP, MEd (Cleveland State University)
Summary: This project aims to help more people of color take part in clinical research so that we can bring more equity to preventing and treating illness. Community health workers (CHWs) and researchers will explore ways to increase participation in clinical trials by people who are Black. Interviews with CHWs and community members will help us plan future projects in which CHWs help engage urban Black community members in meaningful, trustworthy research.
2. Elimination of Black Women's Barriers to Clinical Research on Postpartum Post-Traumatic Stress Disorder
Team:
- PI: Erika Kelley, PhD (University Hospitals)
- Co-I: Sally MacPhedran, MD, MS (MetroHealth), Vidya Visvabharathy, MD (MetroHealth)
- Community Co-I: Veranda Rodgers, MBA, Executive Director, Pregnant with Possibilities Resource Center
Summary: In this observational study, we are examining strategies to mitigate two barriers to clinical trial participation in Black postpartum women: mistrust and lack of access to clinical trial information. We will compare women's participation rates in a survey based on: (a) recruitment hospital-based flyers vs. a peer-led informational session at a community based, trusted center (Pregnant with Possibilities Resource Center; PPRC), and (b) location of survey completion in the hospital vs. PPRC.
3. Be INFORMED Community Wellness Project
Team:
- PI: Carla Harwell, MD (University Hospitals)
- Co-I: Katherine Dignan, MS (VA)
- Community Co-I: Stacey Easterling, MPH, National Institute for African American Health
- Study Team: Lena Grafton, PhD, MPH, CHES (University Hospitals), Regime Willis, CHES
Summary: Cleveland is home to over 16,900 veterans with 46.6% of its total population identifying as African American. Despite the size of these populations and access to renowned research institutions, both are underrepresented in clinical trials. This project aims to increase understanding of research within these populations by providing educational sessions and surveys to build trust within research and healthcare systems.
4. Trials-Within-Cohort with Semi-Structured Interviews to Identify Health Goals of Older Men with Complex Multimorbidity and Test Supports to Improve Clinical Trial Engagement
Team:
- PI: Holly Hartman, PhD (CWRU)
- Co-I: Randy Vince Jr., MD, MS (University Hospitals), Sarah Koopman-Gonzalez, PhD (CWRU)
- Community Co-I: Laura Kleinman, Senior Transportation Connection
Summary: We will recruit older men that have several medical problems and few resources. We will interview them to learn about their health goals and barriers to joining clinical trials. When coming in for the interview, we will randomize them so that only one group receives additional support (e.g. transportation) to test ways to make it easier to join a clinical trial. We also want to show our study design, called trials-within-cohort, is a good way to test methods to engage people in clinical trials.
5. Community Engagement in the Development of an Abbreviated Mindfulness-Based Cognitive Therapy Intervention for Depression in Older African American/Black Breast Cancer Survivors
Team:
- PI: Jacob Hill, ND (Cleveland Clinic)
- Co-I: Nora Nock, PhD (CWRU)
- Community Co-I: Sydney Beeman, MA, NCC, LPC, The Gathering Place
Summary: Older African American/Black (AA/B) breast cancer survivors (BCS) have high rates of depression. Depression increases the risk of cancer returning and death. Medicines for depression don't always work and have side effects, so new treatments are needed. However, older AA/B individuals are often not included in research, and little is known about new ways to treat depression in older AA/B BCS. This study will test a new mindfulness program designed for older AA/B BCS with depression.
6. Reducing Barriers for Hispanic and Latina Patients with Breast Cancer Receiving Radiation Therapy
Team:
- PI: Shearwood McClelland, MD (University Hospitals)
- Co-I: Abizairie Sanchez-Feliciano, MS (CWRU
- Community Co-I: Dan Radocaj, El Centro De Servicios Sociales, Inc.
- Study Team: Chesley Cheatham (University Hospitals)
Summary: Our central hypothesis is that Hispanic-American breast cancer patients offered RT with navigator-assistance (including education regarding efficacy of short courses when eligible and transportation assistance for short and long courses) will be more likely to complete radiation than patients without navigator and transportation assistance. We propose a series of studies to assess barriers to patient navigator access (Aim 1), the impact of patient navigation on RT access (Aim 2), and investigating financial toxicity differences between standard versus hypofractionation faced by Hispanic-American breast cancer patients (Aim 3).
7. Realizing the Net Benefit of Newborn Screening: Addressing Racial Disparities in Awareness, Return of Results, and Time to Treatment
Team:
- PI: Lynette Gerido, PhD, MPH, MBA (CWRU)
- Community Co-I: Angela Newman-White, MS, First Year Cleveland
- Study Team: Aaron Goldenberg, PhD (CWRU), Marsha Michie, PhD (CWRU)
Summary: Newborn screening (NBS) saves lives by identifying rare but serious medical conditions before symptoms present and treating them quickly. We will partner with home-based health professionals (i.e., nurses, midwives, etc.) involved in prenatal and newborn care to identify community-led education and actions that can be implemented during prenatal care or the first year of life to reduce racial disparities in infant mortality and raises awareness of related rare disease clinical trials.
8. Elite Youth Enrollment Better Together Program: Focus on Mental Health and Clinical Trials
Team:
- PI: Edward Barksdale, MD (University Hospitals)
- Co-PI: Andrew Pollis, JD (CWRU)
- Community Co-I: Robert Combs, Elite Youth Enrollment
- Study Team: Pamela Combs, CNP (Cleveland Clinic), LaShelle Henderson, Mary Louise Tatum, MPH, MSN, PMHNP-BC (Cleveland Clinic), Sean Shenker, Jorell Chambers, Aldonte Flonnoy, Cheryl Combs
Summary: This project will reduce the barriers to participation in clinical trials among African American males aged 18-25 by sharing information about the safety, efficacy, and importance of clinical trials as well as by surveying participants to better understand the barriers unique to the target population so they are eliminated. Further, the project will aid participants in exploring careers that support the facilitation of clinical trials to increase awareness and potential career development.
9. Achieving Health Equity: Recognizing the Health Profiles of Asian American and Pacific Islander Communities and Closing the Gap in Clinical Trials
Team:
- PI: Ye-Fan W. Glavin, PhD (CWRU)
- Co-PI: Prakash Ganesh, MD (University Hospitals)
- Co-I: Andre L. Brown, PhD, MPH, Cuyahoga County Board of Health,
- Co-I: Minzhi Ye, PhD, Kent State University
Summary: Cuyahoga County is home to both a thriving Asian American and Pacific Islander (AAPI) population as well as world-renowned medical research institutions. Despite this proximity, the county's AAPI population is underrepresented in clinical trials. This project will build on a partnership with the AAPI Health Coalition to access multiple AAPI ethnic groups to facilitate culturally competent dialogues and surveys to identify barriers to clinical trial participation unique to the population.
10. HOLA Ohio Project
Team:
- PI: Debora Bruno, MD (University Hospitals)
- Co-PI: Monica Yepes-Rios, MD (Cleveland Clinic)
- Community Co-I: Veronica Dahlberg, HOLA Ohio
Summary: HOLA Ohio will engage with Hispanic workers, families, and children in Lake and Ashtabula counties to better understand the barriers that inhibit this target population's participation in clinical trials. To do so, it will facilitate informational sessions for families to close gaps in knowledge and identify barriers to participation. In addition, the project will provide a STEAM-based enrichment and tutoring program for students in grades K-12 that is informed by research career fields.
--