feature
Going Deep in the Brain
by Andrea Appleton
 photo: Roadell Hickman
Cameron McIntyre
photo: Roadell Hickman
Cameron McIntyre
In the late 1990s, Cameron McIntyre was well on his way to becoming a double alum of Case Western Reserve by adding a doctorate in biomedical engineering to the bachelor's degree he'd already earned.
He'd found his research focus: the biophysics of the interaction between electric fields and neurons. Yet he still felt professionally adrift.
"I was trying to figure out something useful to do with that knowledge," he says.
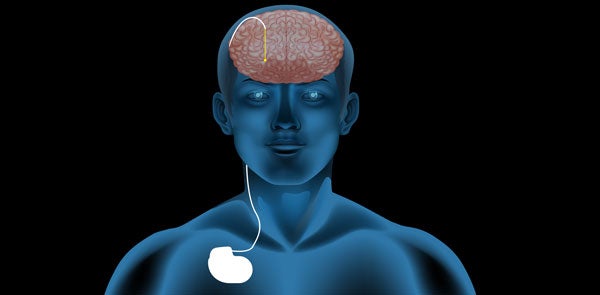
One day, he went to a presentation about a clinical therapy called deep brain stimulation (DBS) that was just making inroads in the United States. The therapy, primarily used to treat symptoms of Parkinson's disease, called for surgically implanting a stopwatch-sized pacemaker under the clavicle. Insulated wires (known as leads) carried electrical stimulation from the pacemaker to the basal ganglia, a region of the brain associated with the planning of movement. The results were unprecedented: Tremors and rigidity melted away, dexterity improved, and some people who struggled to take even a step could walk again.
"I was just blown away by the whole thing," McIntyre says. "So I said, 'This is what I'm gonna do.' And I've been working on it ever since."
After nearly a decade at Cleveland Clinic, McIntyre is now the Tilles-Weidenthal Associate Professor of Neurology at Case Western Reserve, heading a lab dedicated to improving the clinical application of DBS.
Despite the advances made in DBS during the last 20 years, researchers still don't completely understand how the therapy works. Many scientists believe the electrical pulses the system gives off stimulate neurons to fire in response, leaving them too busy to send off the abnormal signals that cause Parkinson's symptoms. But this theory has not been proven definitively, and researchers cannot explain why DBS is less effective with some symptoms and some patients. As a result, tailoring the treatment to a particular patient involves a surprising amount of trial and error.
McIntyre hopes a new software program he and his colleagues are developing in his lab will change that. If he's right, DBS could one day help more patients and provide better relief for those who already have experienced its power.
UNDERSTANDING THE SCIENCE
In Parkinson's patients, neurons in the brain that produce the chemical dopamine die off. The lack of dopamine appears to send the neural pathways between the basal ganglia and the motor cortex—the part of the brain that most directly controls movement—off-kilter, resulting in symptoms that include tremors, rigidity and spasms. (Patients also experience some non-motor symptoms, such as anxiety and dementia.)
"The basal ganglia is a lot like an autopilot," says Ben Walter, MD, director of Case Western Reserve's Movement Disorder Center and medical director of its DBS program. "It allows us to chew gum and walk at the same time—those kinds of automatic movements we take for granted every day."
Before deep brain stimulation, the surgical creation of a lesion in the brain was the treatment of last resort for Parkinson's patients. In the 1990s, DBS came into use. The U.S. Food and Drug Administration since has approved it to treat Parkinson's and several other movement disorders. Researchers now are looking into using DBS to treat other ailments that may involve neural miscommunication, from neuropsychiatric disorders to Alzheimer's disease.
More than 100,000 people worldwide have had a DBS system implanted, and that number is growing quickly. As a bioengineer behind the scenes, McIntyre does not deal directly with patients. But sometimes he's in the operating room, and he works closely with Walter and Jonathan Miller, MD, assistant professor of neurosurgery at the School of Medicine and director of the Center for Functional and Restorative Neurosurgery at University Hospitals Case Medical Center.
"The clinician will tell me what the problems are, or what the questions are," McIntyre says. "But you have to observe it yourself before you really understand what they're asking of you."
McIntyre learned from such collaborations that while the surgery itself has grown ever more precise, subsequent treatment often relies on clinical intuition. After surgery—and on visits throughout the patient's life—a neurologist programs the DBS system, customizing it for the patient.
"We're talking about millimeters here, very fine adjustments," McIntyre says. Voltage and frequency can be changed, and individual electrodes—points on the leads in the brain that stimulate neurons—can be on or off, among many other variables. Because Parkinson's is degenerative, symptoms also change over time. The only way neurologists know if they've hit the right combination is to observe the patient.
"Our big goal was to make that more of a science project as opposed to an art project," McIntyre says.
About a decade ago, he and his team created a computer program that takes some of the guesswork out of the neurologist's task. The program simulates in 3-D what an individual patient's brain looks like with the device implanted, and provides a visual target both for surgeons putting the wires into the brain and for neurologists adjusting the electrical dosage for stimulation post-surgery.
GUIDING SOFTWARE
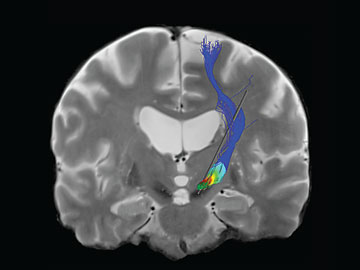
McIntyre’s software program provides a visual image for doctors and scientists that shows the pathways in the brain that electrical stimulation would activate. The color represents the voltage on the pathway.
Research associate Angela Noecker pulls the program up on her computer. Two intersecting planes of a study subject's brain, derived from MRI and CT scans, swivel in front of her. Several 3-D blobs in fluorescent colors hover over the image, indicating specific substructures of the basal ganglia. The area of electrical stimulation—a red glow surrounding a cartoon electrode—grows and shrinks as Noecker clicks her mouse, changing stimulation variables. Finally, the glow matches up with a gray target area on one of the blobs.
"This is where your good-outcome patients are being stimulated," she says.
The system from McIntyre's lab was commercialized, and later became a product of Boston Scientific. It has received regulatory approval for use in Europe.
Now McIntyre is developing a much more sophisticated version. It has the potential for truly individualized treatment for Parkinson's patients. One day, with the help of this software, a patient with tremors could receive stimulation in one area, while one with trouble walking might receive it millimeters away in another.
The new software being developed relies on a number of different brain scan technologies to help generate a model of different pathways in the brain. To understand how the neurons in those pathways will respond to an electrical field, you first must know what that field looks like in each person's brain once it's been distorted by the complex tissue of the brain. McIntyre uses mathematical modeling, his specialty, to create models of those electrical fields. Once the elements are in place—the shape of the electrical field, the route of a particular pathway, and the likely response of the neurons there—he'll have the makings of a simulator. One major obstacle that remains is figuring out which pathway is associated with which symptoms—say, tremors versus rigidity. McIntyre's lab recently received a $3.4 million grant from the National Institutes of Health to research this very question.
The research team will focus on two pathways and will use the new software to figure out how to stimulate one or the other in each patient. The patients' responses to this treatment should tell the researchers whether they've hit the right pathway. Once the pathways are identified, the complexity of the brain's neural connections will provide new puzzles for McIntyre's own active brain for years to come.
"If we start to be able to piece these things apart, we can try to build a map of how these guys are interacting with each other and what it means," McIntyre says. "It's a supercool problem."
To learn more about Cameron McIntyre, the Tilles-Weidenthal Associate Professor of Neurology, visit mcintyrelab.case.edu.
Read More
Exploring the Brain
-
Cultivating Creativity
Brain scans show power of compassionate coaching.
-
Looking Within
New imaging techniques could spur better treatments.
-
Going Deep in the Brain
Locating best place to deliver electrical stimulation.
-
Restorative Touch
Giving man with prosthetic hand the ability to feel what's in his grasp.
-
Fearless
Alumna Lisa Beth Allen (WRC '83) shares what life is like after traumatic brain injury.
-
Cultivating Creativity
Brain scans show power of compassionate coaching.
-
Looking Within
New imaging techniques could spur better treatments.
-
Going Deep in the Brain
Locating best place to deliver electrical stimulation.
-
Restorative Touch
Giving man with prosthetic hand the ability to feel what's in his grasp.
-
Fearless
Alumna Lisa Beth Allen (WRC '83) shares what life is like after traumatic brain injury.