feature
Looking Within
by Andrea Appleton
 photo: Roadell Hickman
Cindy Arth
photo: Roadell Hickman
Cindy Arth
Cindy Arth has struggled with the symptoms of multiple sclerosis (MS) for more than 20 years. She has muscle spasms and trouble walking, and repeatedly wakes up without vision in one eye. But for one beautiful year, the 48-year-old Cleveland resident had no symptoms at all. Arth remembers the day in 2012 when she first noticed something was different.
"I was able to get dressed without sitting down to put my pants on," she says. "I was so excited, I took them off and tried it again."
Arth's reprieve came after she received a bulked-up infusion of her own stem cells through a joint clinical trial involving Cleveland Clinic, University Hospitals Seidman Cancer Center and Case Western Reserve University School of Medicine.

The trial, which ended in January, was based on the research of Case Western Reserve developmental neurobiologist Robert Miller, PhD, the Allen C. Holmes Professor of Neurological Diseases, and biology professor Arnold Caplan, PhD. In MS, the immune system attacks the myelin sheaths that surround slender sections of nerve cells in the brain and spinal cord. This fatty, protective insulation helps with transmission of nerve signals. When it's damaged, the nervous system cannot communicate effectively, leading to a wide range of symptoms, from muscle weakness to muddled thinking.
About a decade ago, Miller and Caplan hit upon the idea of using a kind of adult stem cell found in bone marrow—the mesenchymal stem cell, or MSC—to help rebuild damaged myelin. Caplan had worked with the cells since the late 1980s, and knew MSCs seek out damage or inflammation and stimulate repair. Miller and Caplan launched studies of human MSCs in mice and found the results to be, in Caplan's words, "just spectacular." In the 24-person clinical trial that followed, samples of each patient's own MSCs were extracted, cultured to expand their number and re-injected.
The trial that included Arth was a preliminary study to gauge the safety of the treatment. Cleveland Clinic neurologist and lead investigator Jeffrey Cohen hopes a larger trial will begin sometime this year. Arth, for one, is game. "If they asked me, I would definitely do it again," she says.
Though Arth responded positively to the treatment, it's the response of her neurons that researchers long to see. The symptoms MS patients suffer vary tremendously, and diagnosis can take years. If researchers could observe directly the extent of myelin damage in a patient, knowledge of the disease would grow exponentially. Scientists also would know whether a given treatment was likely to show results more quickly. But the anticipated clinical trial—like all MS research with human beings—will be hampered by a very basic problem: The standard brain scanning technology, magnetic resonance imaging (MRI), can't quantify the extent of damage to the myelin itself.

Mark Griswold, PhD, professor of radiology, Yanming Wang, PhD, an associate professor of radiology, and their teams each recently developed new scanning technologies that could address this problem. One technique soon could eliminate the bottleneck in clinical trials. The other not only may help with MS but could entirely upend the world of brain imaging. Indeed, it eventually could pinpoint most any brain disorder.
THE MAGIC MACHINE
Magnetic resonance imaging provides far more detailed information than CT scans or other less advanced measures. But it often doesn't provide the specific information needed to diagnose and track diseases.
In the case of multiple sclerosis, MRI is used to detect scar-like plaques in the brain. Plaques develop in areas where myelin has been damaged and show up as bright white spots in scans. Unfortunately, so do other unrelated phenomena, including swelling and inflammation. As a result, diagnosing MS (and gauging the effectiveness of any given treatment) is notoriously difficult.
 photo: Roadell Hickman
Yanming Wang with a new model of a cyclotron machine awaiting hook-up.
photo: Roadell Hickman
Yanming Wang with a new model of a cyclotron machine awaiting hook-up.
Wang hopes to address that problem with a cyclotron, which he calls "the magic machine."
The cyclotron produces radioisotopes, which are atoms with unstable nuclei. These radioisotopes are delivered through tubes to Wang's lab one floor up, where they are inserted quickly in molecules that bind to specific targets in the body. The pairing that results is a radioactive tracer. Once injected into the body, the tracer travels through the bloodstream and binds to molecules it is designed to target. The radioactive component allows researchers to see where the molecules have bound, using an imaging technology called positron emission tomography, or PET.
PET creates three-dimensional images of functional processes in the body, and thus has broad medical applications. But PET has never been particularly useful for MS. About a decade ago, Wang's team began looking for a molecule that would bind exclusively to myelin for use as an imaging agent.
"We had to screen hundreds of molecules," Wang says. "It has to function like a guided missile, so it can go straight to the target and then light it up."
Last year, Wang and his colleagues announced they'd finally found one. In lab animals, the molecule, known as MeDAS, binds to myelin in the brain and spinal cord—and seemingly little else. Wang collaboratedclosely with MS researcher Miller to use the technology for drug discovery and development. They gave a component of MSCs—the stem cell that seemed to ease Cindy Arth's symptoms—to rats with damaged myelin in the spinal cord. Then they injected the tracer to capture images at different points in time. An analysis of the raw data that accompanied the images told the researchers how much myelin existed in specific regions throughout the spinal cord. Repeated PET scans taken over the course of treatment enabled the researchers to show that the stem cell component did indeed lead to myelin repair.
If Wang's imaging technology works as well in people such as Arth as it does in rats, researchers soon may know whether a given treatment is working before patients feel a change in symptoms. Wang emphasizes that the same PET imaging technique developed in lab animals often can be directly translated to human beings. "The moment I first looked at the images," he says, "I was fully convinced that it was the one we have been looking for."
A NEW IMAGING TECHNIQUE
Just one floor up from Wang's lab, Mark Griswold and his colleagues are using the basic components of MRI—a magnetic field and radio waves—to create a whole new approach to imaging. MRI is largely subjective, and individual clinicians can interpret the same scan differently. Griswold; Vikas Gulani, MD, PhD, assistant professor of radiology at the School of Medicine and director of MRI at University Hospitals; and their colleagues have worked for more than a decade to develop a technique that would tell users precisely what was happening in a given region of the brain, resulting in a new imaging method called magnetic resonance fingerprinting (MRF).
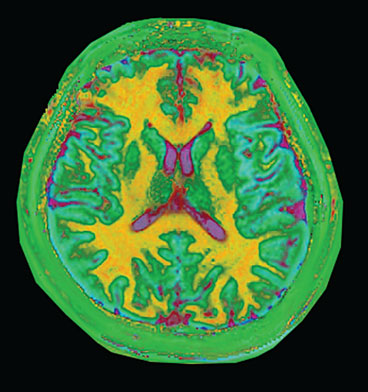 This visualization of magnetic resonance fingerprinting shows different types of brain tissues. White matter (shown as yellow) contains nerve cells insulated by myelin and transmits information between various parts of the brain and to other parts of the body. Gray matter (shown as light blue) contains neural cells and is the repository of memory, emotion and language skills. The cerebrospinal fluid is pink.
This visualization of magnetic resonance fingerprinting shows different types of brain tissues. White matter (shown as yellow) contains nerve cells insulated by myelin and transmits information between various parts of the brain and to other parts of the body. Gray matter (shown as light blue) contains neural cells and is the repository of memory, emotion and language skills. The cerebrospinal fluid is pink.
Researchers already are singing the praises of the MRF innovation. "I think it's going to move us to a whole new level," says Anthony Jack, PhD, an assistant professor of cognitive science, philosophy and psychology who uses a type of MRI in his research. "It's hard to know yet all the things it can do, but it may transform the entire field of MR imaging."
The technique is based on the premise that nearly every tissue in our bodies can be manipulated in a way that creates a unique pattern—or "fingerprint"—in the data received from the MRI scanner. MRF scans for different physical properties simultaneously; the patterns that emerge from a given region then are compared to a dictionary of tissue properties.
Fingerprinting eventually could diagnose neurological disorders by matching the tissue properties measured in a patient's scan to a list of diseases. Preliminary data indicate that researchers already can use MRF to grade different kinds of brain tumors. Fingerprinting also can detect age-related changes in our brains.
"We can guess your age," Griswold says. This isn't just a parlor trick; scans taken periodically throughout a person's life might provide an early indication of degenerative diseases such as Alzheimer's.
Griswold believes fingerprinting eventually could alter the entire imaging field—and, more specifically, assist in diagnoses and treatment of people such as Arth.
"I think we'll have a very early indicator of myelin degradation and progression in MS," Griswold says.
Yes, myelin. Griswold and his colleagues are working closely with MS researchers at Cleveland Clinic, and hope to publish a paper on MS and fingerprinting sometime this year.
In fact, Griswold says, fingerprinting or a similar quantitative process could one day help clinicians diagnose and track nearly every disease that afflicts the brain. "That is the hope," he says.
At the very least, these new PET and MRF imaging technologies could accelerate the pace of MS research. That would be a boon to the 2 million-plus people who suffer from the disease. For that matter, Arth herself could use another reprieve. More than a year has passed since her stem-cell injection, and some symptoms have begun to return.
"I knew that it would not last forever," she says. "But I was hoping it would."
To learn more:
- Longitudinal positron emission tomography imaging for monitoring myelin repair in the spinal cord (wiley.com)
- Magnetic resonance fingerprinting (nature.com)
- About Associate Professor Yanming Wang
- About Professor Mark Griswold
- About Professor Arnold Caplan
Read More
Exploring the Brain
-
Cultivating Creativity
Brain scans show power of compassionate coaching.
-
Looking Within
New imaging techniques could spur better treatments.
-
Going Deep in the Brain
Locating best place to deliver electrical stimulation.
-
Restorative Touch
Giving man with prosthetic hand the ability to feel what's in his grasp.
-
Fearless
Alumna Lisa Beth Allen (WRC '83) shares what life is like after traumatic brain injury.
-
Cultivating Creativity
Brain scans show power of compassionate coaching.
-
Looking Within
New imaging techniques could spur better treatments.
-
Going Deep in the Brain
Locating best place to deliver electrical stimulation.
-
Restorative Touch
Giving man with prosthetic hand the ability to feel what's in his grasp.
-
Fearless
Alumna Lisa Beth Allen (WRC '83) shares what life is like after traumatic brain injury.