|
Background
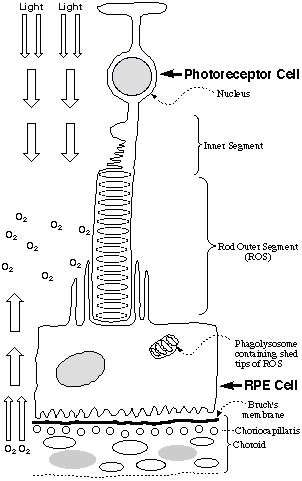 In view of the oxygen-rich environment in the eye, it is particularly noteworthy
that phospholipids containing docosahexaenoic acid (DHA), which are exquisitely
sensitive to oxidative damage (1), are abundant in photoreceptor cells.
These cells contain a stack of DHA-rich membrane disks that are bathed in
oxygen and light (see Figure). A molecule of rhodopsin, a retinylidene
Schiff base of the protein opsin, is embedded in each disk. New disks
are generated at the nuclear end of the stack and old disks are shed at the
tip of the rod outer segment (ROS) where they are digested by phagocytic cells
of the retinyl pigmented epithelium (RPE) (2). To protect against oxidative
damage, the retina contains the antioxidant
a-tocopherol
(vitamin E) and antioxidant enzyme systems including glutathione peroxidase
and superoxide dismutase. Nevertheless, the disks are replaced every
twelve days, probably because they are routinely damaged by oxidative modifications
spawned by photogenerated radicals. Previously, the lipid oxidation
product malondialdehyde was found in the subretinal fluid from individuals
with retinal detachment (3).
In view of the oxygen-rich environment in the eye, it is particularly noteworthy
that phospholipids containing docosahexaenoic acid (DHA), which are exquisitely
sensitive to oxidative damage (1), are abundant in photoreceptor cells.
These cells contain a stack of DHA-rich membrane disks that are bathed in
oxygen and light (see Figure). A molecule of rhodopsin, a retinylidene
Schiff base of the protein opsin, is embedded in each disk. New disks
are generated at the nuclear end of the stack and old disks are shed at the
tip of the rod outer segment (ROS) where they are digested by phagocytic cells
of the retinyl pigmented epithelium (RPE) (2). To protect against oxidative
damage, the retina contains the antioxidant
a-tocopherol
(vitamin E) and antioxidant enzyme systems including glutathione peroxidase
and superoxide dismutase. Nevertheless, the disks are replaced every
twelve days, probably because they are routinely damaged by oxidative modifications
spawned by photogenerated radicals. Previously, the lipid oxidation
product malondialdehyde was found in the subretinal fluid from individuals
with retinal detachment (3).
By acting as antigens that engender
an immune response, oxidatively modified proteins can induce pathological
processes. Some evidence suggests the involvement of the cellular immune
system in retinitis pigmentosa (RP) (4-6), Usher’s syndrome (7), and
cone dystrophy (8). Thus, retinal antigens, especially those localized
in the ROS, foster immune reactivity in RP patients. Although autoimmunity
may not initiate RP, it may contribute to damage of ocular tissues by perpetuating
and maintaining the inflammatory state (9). Antibodies to human retinal
antigens are present in blood serum from RP patients (10-12). One variant
of age-related macular degeneration (ARMD) involves sprouting of new
blood vessels (neovascularization) from the choriocapillaris (see Figure)
into the subretinal space. Large amounts of immunoglobins and complement
components were found in subretinal neovascular membranes from ARMD patients
(13, 14). Very little is known about the molecular structures of retinal
antigens. One possibility is that an immune response may result from
a defect in maintaining tolerance for self-antigens, e. g., rhodopsin.
However, another possibility is that the presence of an abnormally high level
of oxidative protein modifications engenders an immune response.
Oxidative modification of photoreceptor
disk proteins or membrane lipids may also be involved in recognition of damaged
disks by RPE cell CD36 receptors that mediate endocytosis of ROS tips (15).
In analogy with the recognition of oxLDL by macrophage CD36 receptors, it
seems reasonable to expect that modifications of ROS protein or lipids by
products from the free radical-induced oxidation of DHA may be involved in
recognition of damaged ROS disks by CD36. We also recently demonstrated
that a family of oxidized phospholipids derived from linoleate or arachidonate
are CD36 receptor ligands. Structurally similar oxidized phospholipids
may contribute to CD36 receptor recognition of oxidatively damaged ROS.
|
(1) |
Farnsworth, C. C. and Dratz, E. A. (1976) Oxidative damage of retinal rod
outer segment membranes and the role of vitamin E. Biochim. Biophys. Acta
443 556-70. |
|
(2) |
Forrester, J., Dick, A.,
McMenamin, P. and Lee, W. (1996) The Eye Basic Sciences in Practice, WB Saunders,
London. |
|
(3) |
Grattagliano, I., Vendemiale,
G., Boscia, F., Micelli-Ferrari, T., Cardia, L. and Altomare, E. (1998) Oxidative
retinal products and ocular damages in diabetic patients. Free Radic. Biol.
Med. 25 369-72. |
|
(4) |
Brinkman, C. J., Pinckers,
A. J. and Broekhuyse, R. M. (1980) Immune reactivity to different retinal
antigens in patients suffering from retinitis pigmentosa. Invest. Ophthalmol.
Vis. Sci. 19 743-50. |
|
(5) |
Heredia, C. D., Vich,
J. M., Huguet, J., Garcia-Calderon, J. V. and Garcia-Calderon, P. A. (1981)
Altered cellular immunity and suppressor cell activity in patients with primary
retinitis pigmentosa. Br. J. Ophthalmol. 65 850-4. |
|
(6) |
Kumar, M., Gupta, R. M.
and Nema, H. V. (1983) Role of autoimmunity in retinitis pigmentosa. Ann.
Ophthalmol. 15 838-40. |
|
(7) |
Newsome, D. A. and Nussenblatt,
R. B. (1984) Retinal S antigen reactivity in patients with retinitis pigmentosa
and Usher's syndrome. Retina 4 195-9. |
|
(8) |
Isashiki, Y., Ohba, N.,
Nakagawa, M. and Miyake, Y. (1992) Antibodies against human retinal proteins
in serum from patients with cone dystrophy. Jpn. J. Ophthalmol. 36 323-30. |
|
(9) |
Rahi, A. H. and Addison,
D. J. (1983) Autoimmunity and the outer retina. Trans. Ophthalmol. Soc. U.
K. 103 428-37. |
|
(10) |
Chant, S. M., Heckenlively,
J. and Meyers-Elliott, R. H. (1985) Autoimmunity in hereditary retinal degeneration.
I. Basic studies. Br. J. Ophthalmol. 69 19-24. |
|
(11) |
Heckenlively, J. R., Solish,
A. M., Chant, S. M. and Meyers-Elliott, R. H. (1985) Autoimmunity in hereditary
retinal degenerations. II. Clinical studies: antiretinal antibodies and fluorescein
angiogram findings. Br J Ophthalmol 69 758-64. |
|
(12) |
Rahi, A. H. (1973) Autoimmunity
and the retina. II. Raised serum IgM levels in retinitis pigmentosa. Br. J.
Ophthalmol. 57 904-9. |
|
(13) |
Baudouin, C., Peyman,
G. A., Fredj-Reygrobellet, D., Gordon, W. C., Lapalus, P., Gastaud, P. and
Bazan, N. G. (1992) Immunohistological study of subretinal membranes in age-related
macular degeneration. Jpn. J. Ophthalmol. 36 443-51. |
|
(14) |
Lopez, P. F., Grossniklaus,
H. E., Lambert, H. M., Aaberg, T. M., Capone, A., Jr., Sternberg, P., Jr.
and L'Hernault, N. (1991) Pathologic features of surgically excised subretinal
neovascular membranes in age-related macular degeneration. Am. J. Ophthalmol.
112 647-56. |
|
(15) |
Ryeom, S. W., Sparrow,
J. R. and Silverstein, R. L. (1996) CD36 participates in the phagocytosis
of rod outer segments by retinal pigment epithelium. J. Cell Sci. 109 387-395. |
Carboxyethylpyrroles
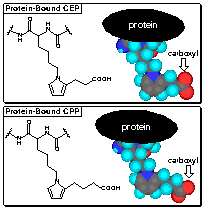 In analogy with the formation of caboxypropylpyrroles
(CPPs) from arachidonyl phospholipids and LDL protein, we postulated the
formation of carboxyethylpyrroles (CEPs) from docosahexanoyl phospholipids
and retinyl protein. Remarkably selective anti-CEP polyclonal rabbit
antibodies were raised that show less than 1% crossreactivity with the analogous
CPPs. Since DHA is the only common polyunsaturated fatty acid whose
oxidation can lead to the production of CEPs, anti-CEP antibodies are a unique
tool for detecting oxidative damage of lipids containing DHA.
In analogy with the formation of caboxypropylpyrroles
(CPPs) from arachidonyl phospholipids and LDL protein, we postulated the
formation of carboxyethylpyrroles (CEPs) from docosahexanoyl phospholipids
and retinyl protein. Remarkably selective anti-CEP polyclonal rabbit
antibodies were raised that show less than 1% crossreactivity with the analogous
CPPs. Since DHA is the only common polyunsaturated fatty acid whose
oxidation can lead to the production of CEPs, anti-CEP antibodies are a unique
tool for detecting oxidative damage of lipids containing DHA.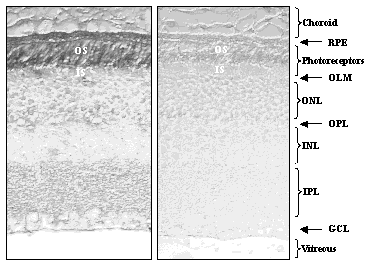
Immunostaining of mouse retina with
anti-CEP antibodies (left panel) was prominant only for the rod outer segments
(OS) and retinyl pigmented epithelium (RPE). As predicted, CEPs are
only generated in DHA-rich regions of the retina. The antibody binding
is specific since immunostaining was blocked if the antibodies were preincubated
with a CEP-containing protein (right panel).
Ongoing studies are directed at identifying
specific proteins in the ROS-RPE proteome that contain CEP modifications using
MALDI-TOF mass spectroscopic analysis of immunoreactive proteins after separation
by two dimensional gel electrophoresis. To further enhance specificity,
monoclonal antibodies are being prepared. The possibility that oxidized
lipids from the retina can be detected as CEP modifications of blood proteins
is receiving special attention because this could provide a clinically useful
tool for the early detection of oxidative injury of the retina.
- Group Contact: C. Charvet
- Collaborators: J. Crabb, J. Hollyfield, I. Pikuleva
Oxidatively
Modified Ethanolamine Phospholipids
A large proportion of the lipids in
photoreceptor disk membranes are contained in ethanolamine phospholipids.
In analogy with the oxidative cleavage of
arachidonyl phosphatidylcholine, we expect that oxobutyryl (OB), succinyl
(S) and hydroxy-7-oxoheptenoic acid (HOHA) phosphatidylethanolamine (PE)
esters will be produced by oxidative cleavage of the docosahexaenoic acid
(DHA) ester of PE. HOHA-PE is the putative precursor of CEP
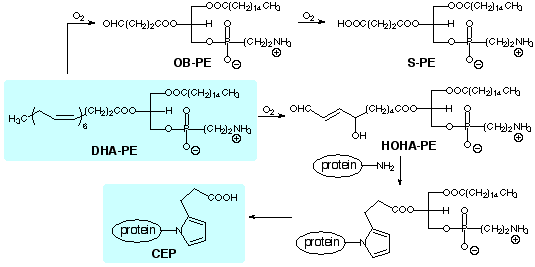
We are preparing samples of these and other
oxadized phosphatidylethanolamines by unambiguous total syntheses to facilitate
their identification and isolation from biological samples, as well as to
allow studies of their biological activities.
|