
Current Group Research
Nitro-polycyclic Aromatic Hydrocarbons
Nitro-polycyclic aromatic hydrocarbons (nitro-PAHs) constitute one of the most important classes of environmental pollutants, found in air, aquatic systems, grilled food, and sediments. Nitro-PAHs are released to the environment as a result of direct emissions from incomplete combustion processes and are formed in situ in the atmosphere by gas phase oxidation and nitrite (NO3) radical reactions of PAHs. Many nitro-PAHs have been identified as mutagenic and carcinogenic agents, and continued concern about these compounds derives from the potential risk they pose to human health.
Photochemical degradation is by far the most important process of natural removal of nitro-PAHs in the environment. Consequently, the investigation of the excited states relaxation pathways is a necessary first step to understand its photochemical fate in the environment and to develop effective atmospheric pollution control strategies.
This program is sponsored in part by the donors of the American Chemical Society Petroleum Research Fund.
Example of the Ultrafast Spectroscopy of 1-Nitropyrene
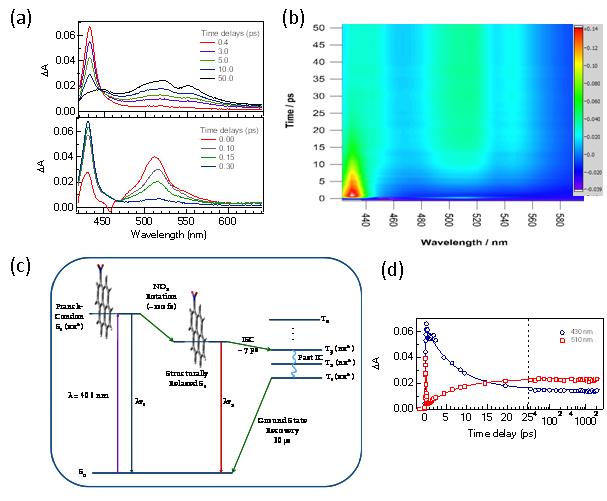 |
Figure legend: Femtosecond broadband transient absorption spectra for 1-nitropyrene (1NP) in cyclohexane solution after photoexcitation at 406 nm: (a) first 0.30 ps (bottom panel) and from 0.4 to 50 ps (top panel). (b) Contour plot representation of 1NP excited-state dynamics. (c) Proposed excited-state relaxation mechanism in nonpolar solvents. (d) Representative transient decay signals at 430 and 510 nm probe wavelengths. See Crespo-Hern�ndez et al. J. Phys. Chem. A 2008, 112, 6313 for details.
Recent Publications
- Brister, M. M.; Piñero-Santiago, L. E.; Morel, M.; Arce, R.; Crespo-Hernández, C. E., "Photochemical Relaxation Pathways in Dinitropyrene Isomer Pollutants", J. Phys. Chem. A 2017, 121, 8197-8206. Access article
- Brister, M. M.; Piñero-Santiago, L. E.; Morel, M.; Arce, R.; Crespo-Hernández, C. E., "The Photochemical Branching Ratio in 1,6-Dinotropyrene Depends on Excitation Energy", J. Phys. Chem. Lett. 2016, 7, 5086-5092. Access article
- Crespo-Hernández, C. E.; Vogt, R. A.; Sealey, B.;† "On the Primary Reaction Pathways in the Photochemistry of Nitro-Polycyclic Aromatic Hydrocarbons", Mod. Chem. Appl. 2013, 1, 106. † Fellow of the ACS Project SEED Program for economically disadvantage high school students. doi: 10.4172/ 2329-6798 .1000106 or Access article
- Vogt, R. A.; Reichardt, C.; Crespo-Hernández, C. E.; "Excited-State Dynamics in Nitro-Naphthalene Derivatives: Intersystem Crossing to the Triplet Manifold in Hundreds of Femtoseconds", J. Phys. Chem. A 2013, 117, 6580-6588. Access article
- Vogt, R. A.; Crespo-Hernández, C. E.; "Conformational Control in the Population of the Triplet State and Photoreactivity of Nitronaphthalene Derivatives", J. Phys. Chem. A 2013, 117, 14100-14108. Access article
- Reichardt, C.; Vogt, R. A.; Crespo-Hern�ndez, C. E.; "On the Origin of Ultrafast Nonradiative Transitions in Nitro-PAHs: Excited-State Dynamics in 1-Nitronaphthalene", J. Chem. Phys. 2009, 131, 224518.Access article
- Vogt, R. A.; Rahman, S.; Crespo-Hern�ndez, C. E., "Structure-Activity Relationships in Nitro-Aromatic Compounds", In Practical Aspects of Computational Chemistry. Methods, Concepts and Applications, Leszczynski, J.; Shukla, M. K., eds., Springer, Netherlands, 2009, pp. 217-240.Access article
- C. E. Crespo-Hern�ndez; Burdzinski, G.; Arce, R.; "Environmental Photochemistry of Nitro-PAHs: Direct Observation of Ultrafast Intersystem Crossing in 1-Nitropyrene", J. Phys. Chem. A 2008, 112, 6313-6319. Access article