Our lab's research primarily focuses on the intersection of biomaterials, immune engineering, and immunometabolism. Our work aims to develop novel biomaterials for immunotherapies, targeting diseases such as cancer, autoimmune disorders, and chronic inflammatory conditions. We have developed metabolite-based biomaterials to modulate immune cell functions by controlling their energy metabolism. This innovative approach has led to significant advancements in generating robust immune responses in models of multiple sclerosis, traumatic brain injury, rheumatoid arthritis, and melanoma.
Our lab has two main research areas - Immunoengineering and Immunometabolism.
Immune Engineering - Immune engineering is a field that brings together the areas of engineering and immunology to harness the power of the immune system and resolve different diseases. We work on these areas to address cancer, inflammation, injury and autoimmune diseases.
Immunometabolism - Immunometabolism is a field that is at the interface of immunology and metabolism. Metabolic processes play a pivotal role in governing the immune cell responses in both healthy individuals in inflammation, infection, cancer, autoimmune disorders, and obesity. Notably, metabolites originating from the mammalian cells, microbiota and infectious agents influence the behavior of the immune cells and overall immune responses.
Based on these ideas, our lab is heralding innovative treatments for chronic inflammatory conditions, autoimmune diseases, and cancer by developing novel immunotherapies. Acharya Lab works on these areas to address a variety of diseases including melanoma, ovarian cancer, traumatic brain injury, rheumatoid arthritis, osteoarthritis, weight loss, and pain dynamics, among other areas.
Our long-term goals are to develop translational biomaterials that can engineer the metabolism of immune cells and develop immunotherapies. To achieve these goals, we develop biomaterials that can effectively modulate the function of immune cells by modulating different metabolic pathways and affecting disease outcomes.
A few example projects -
Immunometabolism Modulating Formulations for Cancer Immunotherapy (Inamdar et al., Suresh et. al)
Our lab focuses on modulating metabolism of innate immune cells (such as dendritic cells, macrophages, etc.) to generate metabolically fit immune cells for cancer vaccines. For example, we have generated succinate based polymers as adjuvants to generate robust immune responses. We have also developed subcutaneous vaccine mouse models and adoptive cell therapy of dendritic cells. This vaccine consists of the metabolite fructose 1,6 bisphosphate that accelerates glycolysis, poly(I:C) as an adjuvant and TRP2 as the melanoma tumor antigen. Moreover, we also develop metabolite based formulations to accelerate metabolism of adaptive T cells to affect ovarian cancer immunotherapy.
References - Nature Communications 14 (1), 5333, 2023 - https://doi.org/10.1038/s41467-023-41016-z.
Biomaterials 301, 122292, 2023 -https://doi.org/10.1016/j.biomaterials.2023.122292
Journal of Controlled Release 358, 541-554, 2023 - https://doi.org/10.1016/j.jconrel.2023.05.014
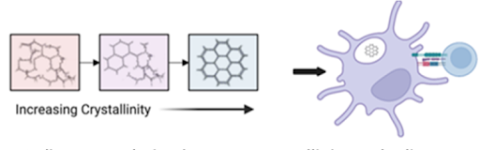
Covalent Organic Frameworks to Understand the Effect of Crystallinity of Biomaterials on Immune Activation (Arezoo Esrafili et al.)
Crystalline biomaterials based on the level of crystallinity may be able to activate the dendritic cells differentially. Shown on the left are schematic of covalent organic frameworks (COFs) with varying crystal structures, and on the right are dendritic cells with a COF affecting downstream T cell responses.
Reference - Crystallinity of covalent organic frameworks controls immune responses, Esrafili A, Thumsi A, Jaggarapu MMCS, Nile RG, Kupfer J, Dugoni M, Suresh AP, Khodaei T, Qian H, Mathis A, Kim B, Swaminathan SJ, Sun W, Seo YW, Lintecum K, Pathak S, Tong X, Holloway JL, Jin K, Acharya AP. Nat Commun. 2024 Nov 11;15(1):9739. doi: 10.1038/s41467-024-54227-9.
Rheumatoid Arthritis Treatment (Abhirami Thumsi et al.; Mangal et al.)
Humans have a recurrence of inflammation termed as flare-ups. This is typically caused by resident T-cells in the tissues. Recapitulating this in mice, we developed a mouse model where the disease can be re-induced by T-cells. We were able to show the efficacy of a metabolite-based modulator- paKG(PFK15+bc2) in prevention of these flare ups in mice.
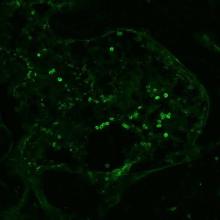
Inflammation leads to an imbalance in energy demand and immune cell profiles in diseases, thereby affecting the normal homeostasis of the body. We aim to evaluate the efficacy of our microparticle- paKG(PFK15+bc2) in restoring homeostasis in collagen induced arthritis in different stages of the disease. We also look at regulatory T cell infiltration in rheumatoid arthritis affected tissue by staining for Foxp3. Shown below is a confocal image of Foxp3+ cells in the paw tissue of a rheumatoid arthritis mouse.
References
Drug Delivery and Translational Research 13 (7), 1925-1935, 2023 - https://doi.org/10.1007/s13346-023-01333-8
Biomaterials Science 10 (23), 6688-6697, 2022 - DOI: 10.1039/D2BM00415A
Biomaterials 277, 121079, 2021 - https://doi.org/10.1016/j.biomaterials.2021.121079
Journal of Materials Chemistry B 8 (24), 5195-5203, 2020 - DOI
https://doi.org/10.1039/D0TB00790K
Improving Cutaneous Wound Healing Rates (Jaggarapu et al.)
This project focuses on polyesters of alpha-ketoglutarate (paKG), generated by reacting alpha-ketoglutarate with aliphatic diols. These paKG particles, developed through an emulsion-evaporation technique, show accelerated keratinocyte wound closures in a scratch assay and faster wound healing responses in mice models. The sustained release of alpha-ketoglutarate from these microparticles presents a promising approach for regenerative therapeutic interventions.
Reference
Journal of Biomedical Materials Research Part A, 2023 - https://doi.org/10.1002/jbm.a.37539
Research Sponsors