Current Research Projects
Epigenetic cell fate conversion for cardiac regenerative medicine
Adult cardiomyocytes (CMs) have limited regenerative capabilities; the loss of CMs due to diseases, such as myocardial infarction (MI), rapidly trigger innate inflammatory response to activate cardiac fibroblasts and often results in chronic heart failure. We had found that fibroblasts in the heart could be directly reprogrammed into induced CM-like cells (iCMs) in vitro and in vivo by a combination of cardiac developmental transcription factors. Currently, we are investigating the mechanism of direct cardiac reprogramming and extending our study from acute MI model to chronic MI model, which will facilitate to translate the nascent technology of cardiac reprogramming into a practical therapeutic paradigm for cardiac regenerative medicine.
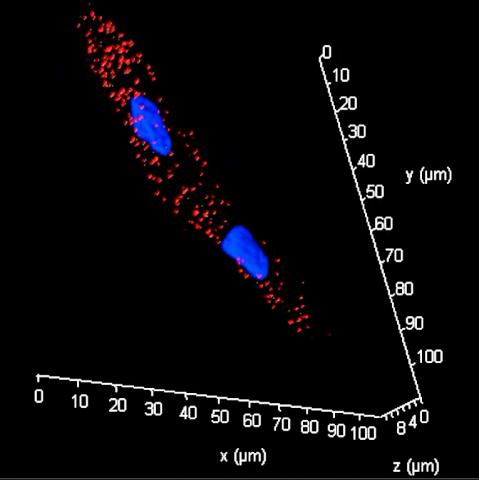
The video below is a 3D reconstructed image of an induced cardiomyocyte (iCM) reprogrammed from a human fibroblast and stained with a marker of the sarcomere:
Development of cardiac electrophysiology and automaticity
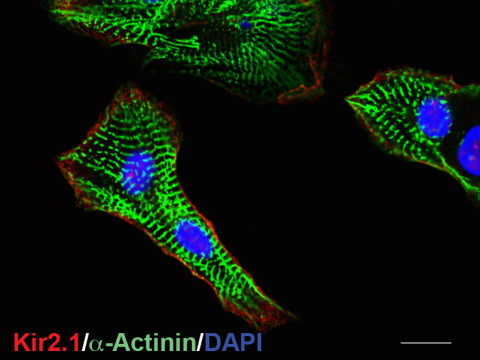
Cardiac arrhythmias, a leading cause of morbidity and mortality, are associated with acquired heart diseases due to profound dysregulation of numerous ion channels and transporters. Both proper expression and appropriate function of cardiac ion channels are obviously required to maintain normal cardiac excitability. In our laboratory, we had been utilizing a critical research model of stem cells, including mouse and human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), and extensively investigated the electrophysiological properties of undifferentiated stem cells and differentiated ESC/iPSC-CMs, including Ca2+ handling and cardiac automaticity. We found a novel mechanism that only two potassium currents, inward rectifier Current (IK1) and pacemaker current (If), were sufficient to induce pacemaker-like electric activities. We termed it IK1-induced If activation and found that it plays an important role in the development of cardiac automaticity. We have been investigating different strategies to facilitate the functional maturation of ESC/iPSC-CMs, with a major goal of improving the efficacy and safety of their clinical applications.
Biophysical regulation of ion channels by microRNAs
MicroRNAs (miRs), small non-coding RNA molecules (~22 nucleotides), are known to post-transcriptionally regulate the protein expression of target genes through mRNA silencing. and are increasingly recognized for their maintenance of normal cardiac function and association with specific disease conditions, including cardiac arrhythmias. In our laboratory, we had investigated the critical roles of miR-1 and miR-499 in regulating the expressions of ion channels and in controlling the differentiation and electrophysiological maturation of ESC-CMs during cardiac differentiation. Very recently, we discovered a novel new function of microRNA that can directly bind with ion channels and biophysically modulate their functions, which is beyond its classical post-transcriptional regulation. This study develops a new research direction of microRNA biology and could have broad implications for multiple cardiac proteins and diseases.