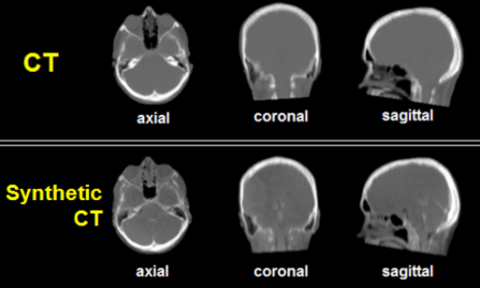
Quantitative PET/MRI Imaging
PET/MR is a hybrid imaging modality that combines the exquisite soft tissue contrast of MR with the molecular information of PET. In order to utilize PET/MR in a clinical trial setting, images must be quantitatively accurate and be reproducible across vendor platforms and institutions. Accurate MR-based attenuation correction (MR-AC) is currently a technical barrier to accomplishing these goals. A specific challenge is differentiating bone from air. While these tissue types have dramatic differences in the degree to which they attenuate photons, they both have negligible signal with conventional MR pulse sequences. Consequently, current MR-AC methods exhibit specific uptake values (SUV) errors of 20 percent or greater—particularly in areas within and adjacent to bone—and therefore, current PET/MR scanners do not meet the SUV accuracy required by the National Cancer Institute/American College of Radiology Imaging Network (NCI/ACRIN) for clinical trials qualification. Ultra-short echo time (UTE) MR can capture signal in bone prior to its rapid signal decay and is a promising approach to achieve more accurate MR-AC. However, current UTE approaches have low image quality, clinically impractical acquisition times, and a field of view that is too limited for whole-body imaging.
Our Goal
The goal of this academic-industrial collaboration is to address these current limitations of UTE by developing accurate and clinically practical methods for whole-body MR-AC, further refining novel and patented methods developed by our working group. We are working in both novel MR acquisition methods and machine-learning image processing to generate synthetic CT images from MR data. The technology development will be tested for clinical feasibility in a cancer patient population. The goal is to achieve SUVs that are within 5 percent of those obtained with PET/CT, the reference standard.
Adaptive Radiation Therapy
Lung cancer remains the leading cause of cancer-related death in the United States. Radiation therapy (RT) is a principal treatment modality used concurrently with chemotherapy in locally advanced disease. Unfortunately, despite improvements in radiation therapy delivery and chemotherapy, overall survival in locally advanced disease remains poor and side effects such as radiation-induced lung injury can be fatal. Further, despite evidence that higher radiation doses cure more patients with non-small cell lung cancer (NSCLC), cure is often limited due to radiation tolerances of normal tissue (heart, lung, spinal cord, etc.) within or adjacent to the radiation field.
What does ART do?
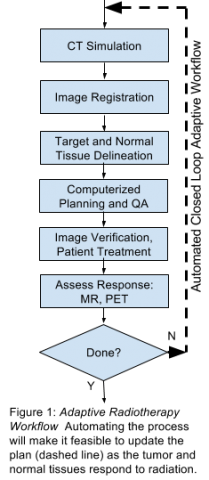
Adaptive radiation therapy (ART)—dynamically modifying the radiation field during the course of treatment as the tumor responds—simultaneously enables increasing the radiation dose delivered to tumor and reduces the radiation dose to normal tissues, improving local tumor control and decreasing normal tissue toxicity. In particular, NSCLC tumors treated radiation shrink by approximately 1 percent per treatment day and 50 percent over six to eight weeks—the full course of treatment.
Developing a Solution
Unfortunately, the ART workflow is too time-consuming and labor intensive for widespread clinical use. To overcome the technical challenges and deliver a clinical solution, the multi-disciplinary team from Case Western Reserve University (CWRU), University Hospitals Seidman Cancer Center (UHSCC), and MIM Software Inc. will extend their existing collaboration to develop a commercial, closed-loop PET- and MR-guided adaptive radiotherapy software suite, which can provide a patient-specific radiotherapy plan in a single day. As radiation oncology departments use a variety of different imaging vendors and scanners, a key focus of this proposal is optimization of quantitative PET and MRI techniques that are widely translatable. The project also leverages in-development technology at CWRU and MIM Software—including image processing methods for MRI-only dose calculations—ultimately obviating the need for CT. Further, the CWRU investigators have expertise in lung imaging with MRI, generating quantitative normalized T1 maps, which can differentiate between functional and nonfunctional lung tissues. Quantitative normalized T1 mapping will be incorporated into radiation treatment planning by preferentially directing the radiation beams through non-functional lung, maximally sparing healthy lung, and potentially decreasing side effects such as radiation-induced lung injury. Additionally, tumor perfusion imaging with PET can help differentiate radiation-induced injury from residual cancer, and when combined with the soft tissue contrasts from MR and machine learning analysis, can be used for automatic segmentation of cancer/target volumes. Finally, the project expands a prototype physician decision support system collaboratively developed by the investigators that assists the radiation oncologist to answer fundamental questions, “which” patients need adaptive radiation therapy and “when” to implement a new radiotherapy plan.
Our Goals
The goals of the proposal are to:
- Implement first pass tumor perfusion quantification using 18FDG-PET for enhanced tumor delineation and automatic segmentation;
- To optimize quantitative lung MRI acquisition techniques that can differentiate lung tissue that is able to oxygenate blood from non-functional tissue so that healthy lung can be maximally spared; and
- To create software suite for PET- and MR- guided adaptive radiation therapy.
We will then demonstrate the clinical feasibility of PET- and MR- guided adaptive radiotherapy in NSCLC in a phase I patient-specific dose-escalated adaptive radiotherapy clinical trial with weekly re-planning based upon image assessment of tumor and normal tissue responses to radiation.