|
 Sodium
Channels play a central role in the transmission of action
potentials along a nerve and are prime targets for methods
to block propagation. Sodium channels are part of a family
of proteins that traverse the cell membrane and form controlled
conduits for the exchange of ions between the intra-cellular
and extra-cellular fluid compartments. These channels are
characterized by their voltage-dependent activation, rapid
inactivation, and selective ion conductances. Sodium
Channels play a central role in the transmission of action
potentials along a nerve and are prime targets for methods
to block propagation. Sodium channels are part of a family
of proteins that traverse the cell membrane and form controlled
conduits for the exchange of ions between the intra-cellular
and extra-cellular fluid compartments. These channels are
characterized by their voltage-dependent activation, rapid
inactivation, and selective ion conductances.
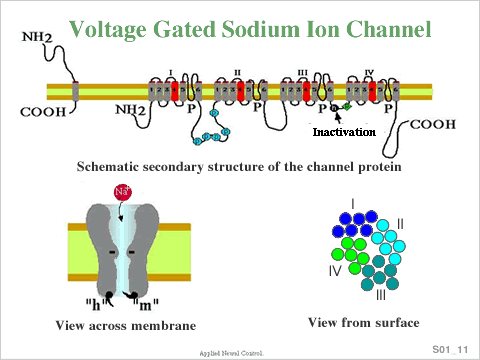
The Voltage Gated Sodium Channel protein consists of four homologous domains
(I–IV), each with six transmembrane segments (S1–S6, Schematic in
upper part of Figure). Single segments and domains are connected by intracellular
and extracellular loops. The hydrophobic S4 segment is found in all voltage gated
ion channels and is absent in ligand gated channels. The four domains are arrayed
in the membrane to form a central pore of ~ 3 to 4 Angstroms (Schematic in lower
part of Figure). Loops between the S5 & S6 segments form an ion selective
filter. The linker between III & IV domains forms the inactivation gate.
The Voltage Gated Sodium Channel can be in different functional states; (1) a
resting state when it can respond to a depolarizing voltage changes (2) activated,
when it allows flow of Sodium ions through the channel, (3) Inactivated – when
subjected to a suprathreshold potential the channel will not open to allow sodium
ions to pass through it.
Historical Note: Because the lipid bilayer of the cell membrane
creates a barrier for passage of the water-soluble ions,
William Bayliss and Ernst Brückehad postulated the existence of pores in the cell membrane. John Langley formulated
the idea of 'receptor molecules' on the cell membrane. In the 1940's, Kenneth
Cole developed the 'voltage clamp' technique
and studies by Alan Hodgkin and
Andrew Huxley established the role of Sodium and Potassium ions in action potential
propagation. This led to the formulation of the Hodgkin-Huxley mathematical models
in the 1950's. In the next decade, Tetradotoxin, a biotoxin was found to prevent
Sodium transfer across the membrane, pointing to a ion selective pathway. In
the seventies, Hladkey and Hayden demonstrated that the antibiotic Gramicidin
could form channels in a lipid bilayer, giving rise to step changes in membrane
current. Hille formulated the idea of a ion selective 'filter' in the channel.
The introduction of the 'patch - clamp' technique by Neher and Sakman enabled
current measurements from single channels. Recombinant DNA technology led to
the isolation, cloning and sequencing of the VGSC by Noda in 1984. An x-ray crystallographic
study of a Potassium ion channel was published by Doyle in 1998.
Review Articles:
From Ionic Currents to Molecular Mechanisms: The Structure
and Function of Voltage-Gated Sodium Channels. Catterall,
W. A. (2000) Neuron, Vol. 26, 13–25.
Structure, Function and Pharmacology of voltage-gated sodium
channels. Denac, H. et. al. (2000) Arch. Pharmacol. 362:
453-479.
|

