Welcome to the Zigmond Lab Webpage!
Lab Research History
In one of the very early examples of activity dependent gene expression, we showed that stimulation of the cervical sympathetic trunk led to an increase in the activity of protein level, and mRNA level of tyrosine hydroxylase, the enzyme that catalyzes the rate-limiting step in the synthesis of the neurotransmitter norepinephrine in the superior cervical ganglion (SCG). Interestingly, antidromic stimulation of the superior cervical ganglion did not mimic the effect of synaptic stimulation.
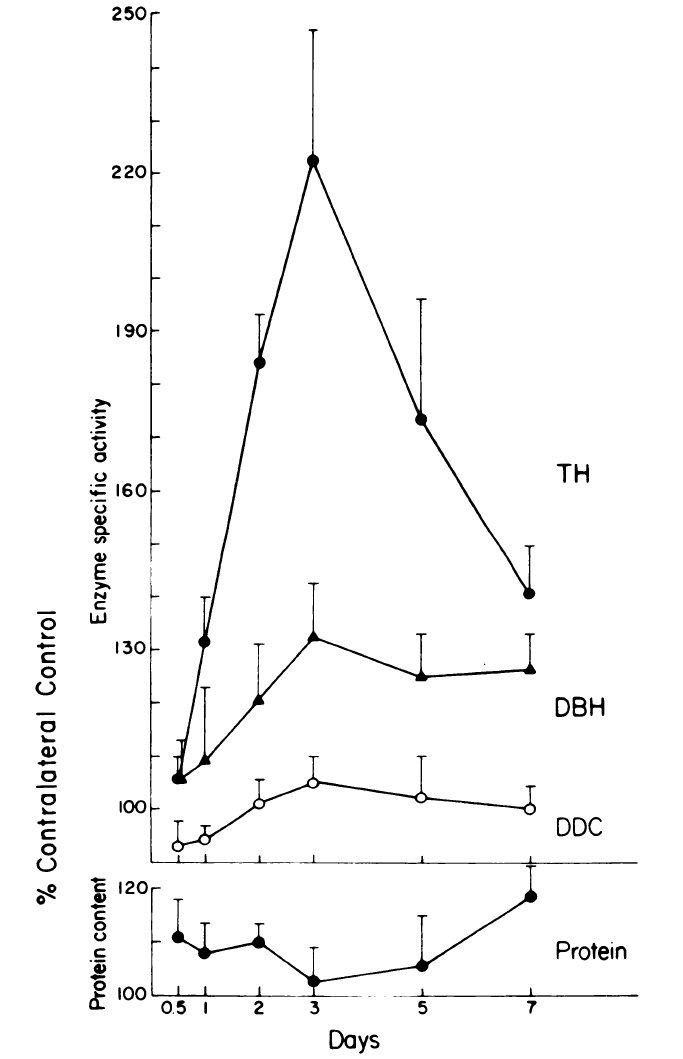
Fig. 1. Time course of the effects of preganglionic nerve stimulation on tyrosine hydroxylase (TH), dopamine beta-hydroxylase (DBH), and dopa decarboxylase (DDC) in the SCG. The cervical sympathetic trunk (CST) was stimulated for 90 min with trains of 40 Hz for 250 out of every 750 msec. TH and DBH activity reached a maximum of 3 days. Neither DDC nor total protein changed significantly. The data are the means +/- S.E.M. from 8-10 SCG except for day 5 on which N=5.
In studies on a second mechanism of TH regulation, namely the acute activation of this enzyme by phosphorylation, we made the unexpected discovery that synapses in the rat SCG use a second transmitted in addition to acetylcholine (Fig. 2), and we presented evidence suggesting that this molecule could be a peptide of the secretin-glucagon family (Fig. 3). Currently evidence suggests that the transmitter is pituitary adenylate cyclase-activating peptide (PACAP).
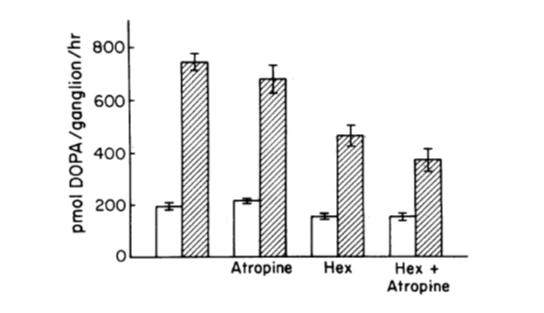
Fig 2. The preganglionic trunk of the SCG was stimulated at 10 Hz for 30 min. Atropine, Hexamethonium (Hex), or both antagonists were added 10 min prior to the beginning of stimulation. Hatched bars stimulate the ganglia, and open bars control the ganglia.
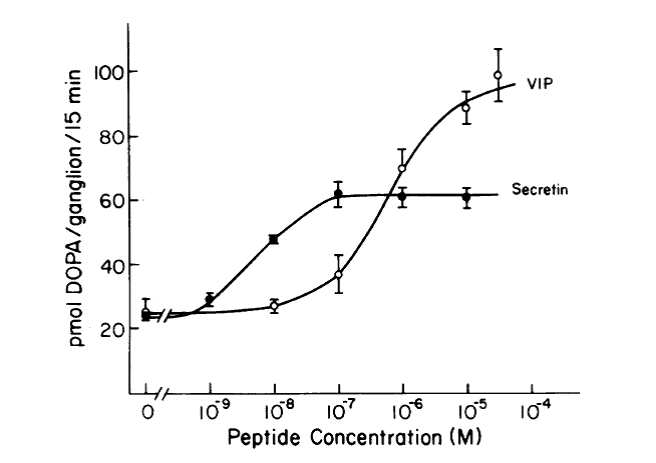
Fig. 3. Concentration-response curve for the stimulation of TH activity by secretin and VIP. Ganglia were preincubated with peptide for 60 min prior to the addition of a dopa decarboxylase inhibitor. Dopa accumulation was measured during the subsequent 15 min.
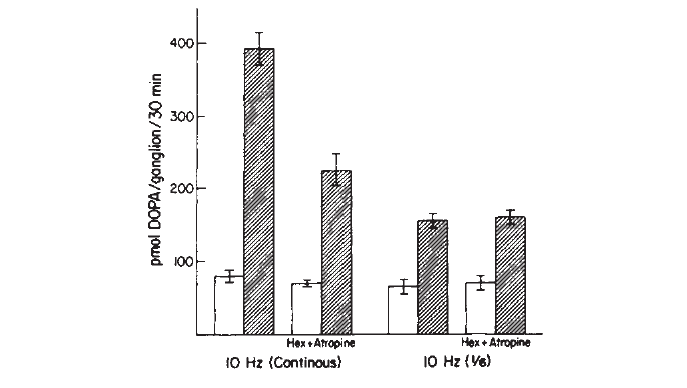
An exciting consequence of the discovery that there is cotransmission in this ganglion was the finding that the extent to which transmission in the SCG is cholinergic or non-cholinergic (peptidergic) depends on the pattern of impulse activity (Fig. 4).
Fig. 4. Effect of cholinergic antagonists on the increase in TH activity produced by preganglionic nerve stimulation. Stimulation was for 30 min at 10 Hz continuously or at 10 Hz for 1 out of every 6 sec. Hexamethonium (3mM) and atropine (6µM) were added to some groups prior to stimulation. The presence of a cholinergic component in the transsynaptic effect depends on the stimulation pattern.
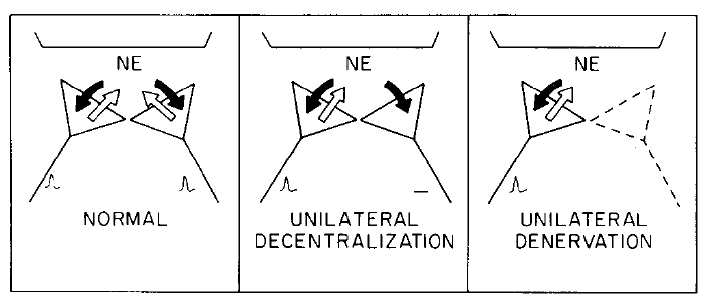
In a second neuronal system, we showed that the 40-fold circadian rhythm in the activity of pineal serotonin N-acetyltransferase (NAT), a regulatory enzyme in the pathway for melatonin synthesis, could be mimicked by electrical stimulation of the sympathetic innervation of the gland (producing a night time effect) followed by cessation of stimulation (producing a day time effect). While studying the pineal gland, we recognized that the rhythm in NAT provided a unique bioassay that could be used for measuring recovery of function following lesions in the sympathetic nervous system. These studies lead us to two important discoveries. The first finding was that of a novel mechanism of neural plasticity, which we termed “hetero-neuronal uptake”, in which a nerve ending (even one that is itself electrically silent) can take up the transmitter released by another ending and thereby modulate the latter’s postsynaptic efficacy (Fig. 5).
Fig. 5. A model to account for the recovery of pineal NAT activity after unilateral denervation (cutting the postganglionic internal carotid nerve) but not after unilateral decentralization (cutting the preganglionic CST). Two sympathetic nerves, one from the left and one from the right SCG are pictured innervating the same pinealocyte. In a normal animal, both neurons are electrophysiologically active and are releasing NE as shown by the white arrows. In addition, both neurons take up NE as shown by the black arrows. The situation in unilaterally decentralized pineal glands is depicted in the middle panel. In this diagram, a neuron from one SCG is shown as electrophysiologically inactive and therefore as not releasing NE. However, the uptake capacity of this neuron is intact. Therefore the net effect of such a neuron is to inhibit the postsynaptic effectiveness of the active neurons from the contralateral ganglion. In the right panel, the situation in the unilaterally denervated pineal gland is shown. Sometime between 9 and 32 h after cutting one postganglionic trunk, the terminals of these nerves degenerate, thus decreasing the number of varicosities taking up NE. The loss of these NE uptake sites increases the postsynaptic effects of the neurons from the contralateral intact ganglion.
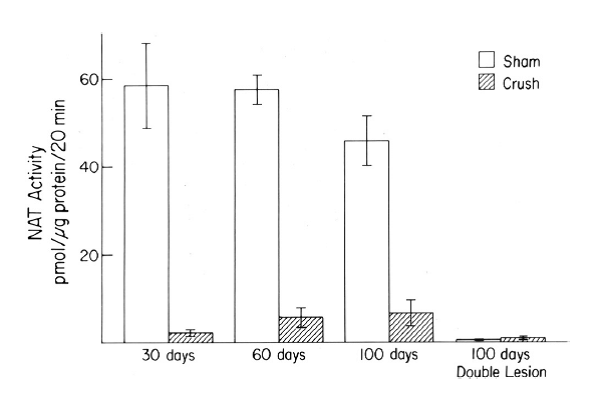
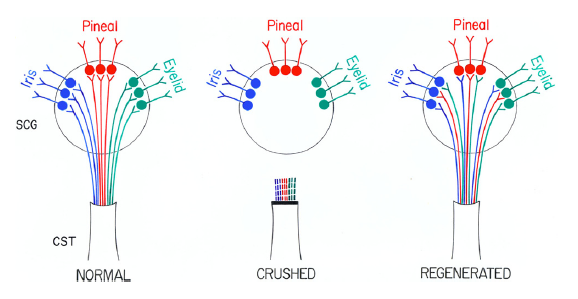
The second finding was that, although preganglionic sympathetic nerves can regenerate after a lesion and can effectively reinnervate neurons in the SCG, normal pineal function is not restored (Fig. 6). This finding conflicts with the prediction from a classical study by John Langley in the early 1900’s on regeneration of these neurons. We hypothesize that many of the neurons that reinnervate neurons in the SCG that project to the pineal gland are different from the original neurons and do not get the needed input from the circadian pacemaker in the suprachiasmatic nucleus of the hypothalamus which directs the normal rhythm in NAT activity (Fig. 7).
Fig. 6. Peak night pineal NAT activity after crushing both CSTs and the effect of a double lesion on this recovered activity.
Fig. 7. Model of misrouting of fibers during reinnervation of the SCG.
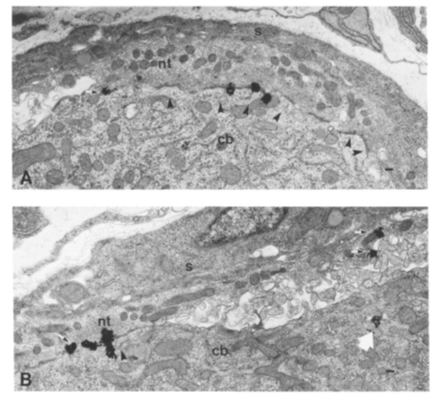
Another unexpected discovery was the finding that the snake venom from which bungarotoxin is purified contains a second toxin (“neuronal bungarotoxin”) which blocks synaptic transmission in autonomic ganglia. After purifying this toxin, we used it to quantify the density of nicotinic receptors at ganglionic synapses. Autoradiographic grains from 125 I-labeled neuronal bungarotoxin were concentrated at synapses in the chick ciliary ganglion as shown in Fig. 8. We also used this labeled toxin to localize a class of nicotinic receptors on SCG neurons in culture and in the rat brain.
Fig. 8. Preganglionic nerve terminals (nt) and post-ganglionic cell bodies (cb) in the chick ciliary ganglion. The black autoradiographic grains from radioactive neuronal bungarotoxin are concentrated at synapses within the ganglion.
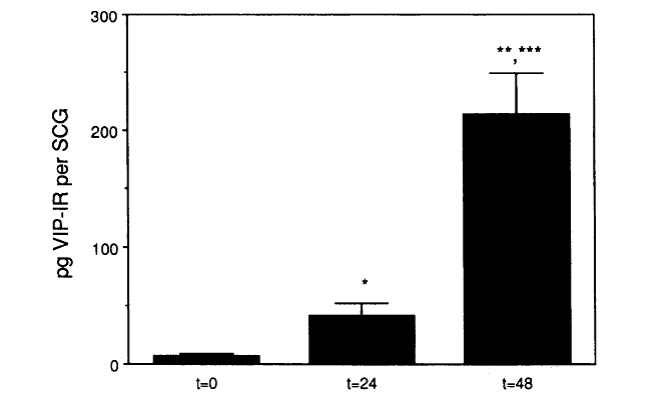
In 1989, I moved to Case Western Reserve University to join its newly formed Department of Neurosciences. During the first year, we made a serendipitous discovery that when the SCG is placed in organ culture levels of vasoactive intestinal peptide (VIP) increase dramatically (i.e., ~30-fold; Fig. 9), rather than decreasing as we expected, since we assumed the peptide was present in preganglionic nerve terminals.
Fig. 9. Increase in VIP-like immunoreactivity in adult SCG maintained in organ culture.
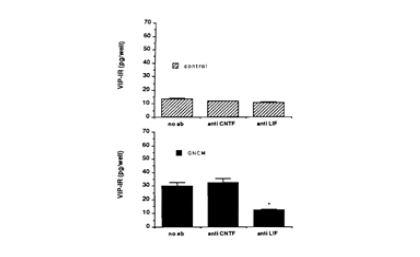
This finding led to the second major theme of our research, namely, the molecular and cellular responses of neurons to injury. When we started these studies, neuropeptide regulation in postganglionic SCG neurons was thought to be regulated primarily by afferent nerve activity, which is abolished in organ culture, the idea being that activity suppressed the peptide phenotype. However, we demonstrated in vivo that it was axotomy of the SCG, not deafferentation, which mimicked the effects seen in organ culture. A trivial reason for this finding would have been that axotomy, by blocking the transport of the peptide, led to a buildup in the cell body. However, we found that axotomy also leads to an increase in VIP mRNA. We went on to find that the cytokine leukemia inhibitory factor (LIF) is induced in non-neuronal cells in the ganglion both after axotomy and after explanation. Conditioned medium from ganglion non-neuronal cells stimulates VIP expression and this effect is blocked by an antibody to LIF (Fig. 10).
LIF, but not CNTF, from non-neuronal cells increases neuronal VIP expression.
Fig. 10. VIP levels in dissociated SCG neurons with and without the addition of ganglion non-neuronal cell conditioned medium (GNCM).
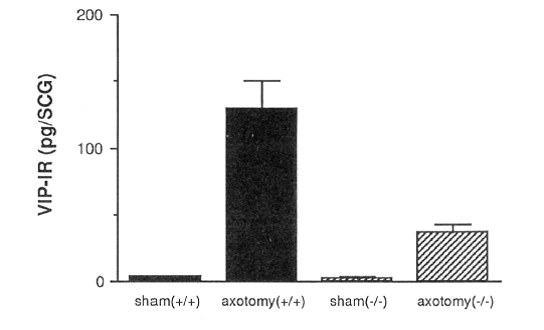
In vivo, the axotomy-induced increase in VIP is reduced, but not completely abolished, in LIF knockout mice (Fig. 11). This finding was the first demonstration of the effect of LIF on the nervous system in vivo. Also, we found that galanin and substance P were regulated similarly.
Fig. 11. Axotomy-induced VIP expression in the SCG is reduced in LIF -/- mice.
Interestingly, unlike the case with the SCG, in the dorsal root ganglion (DRG), LIF was not induced after axotomy; however, the cytokine was induced in the lesioned sciatic nerve. We believe this is the first demonstration of an oft-hypothesized relationship between changes in cytokine and growth factor expression in the distal segment of a lesioned nerve and the expression of certain genes by the axotomized neurons. In addition, using galanin knockout mice, we demonstrated that the induction of galanin after axotomy plays an important role in the conditioning lesion response of sensory neurons.
We have evidence that LIF is only one of a number of molecules that influence the response of sympathetic and sensory neurons to axotomy. LIF is a member of a family of cytokines, called the gp130 family. Also, we have shown that removal of the influence of the target-derived neurotrophic factor nerve growth factor (NGF) is an important signaling event. Injection of animals with a nerve growth factor antiserum produces an increase in galanin expression in both the SCG and DRG and triggers a conditioning lesion-like effect in sympathetic neurons.
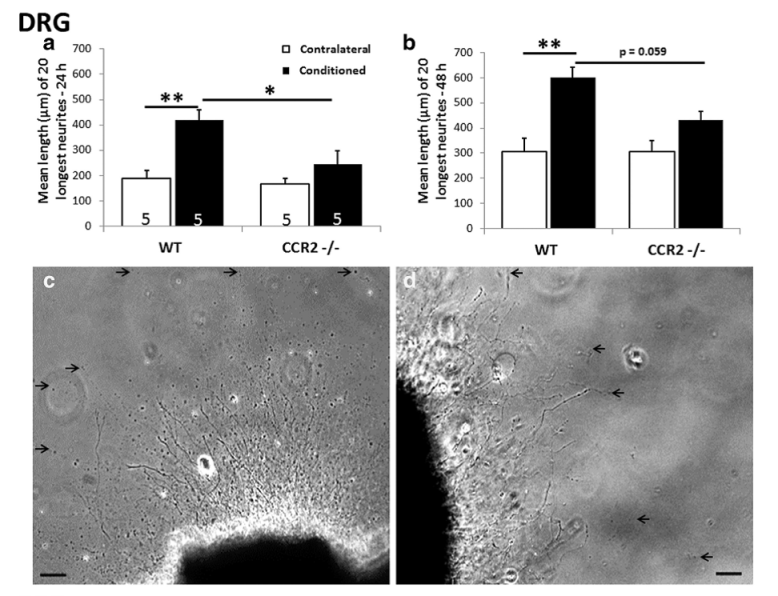
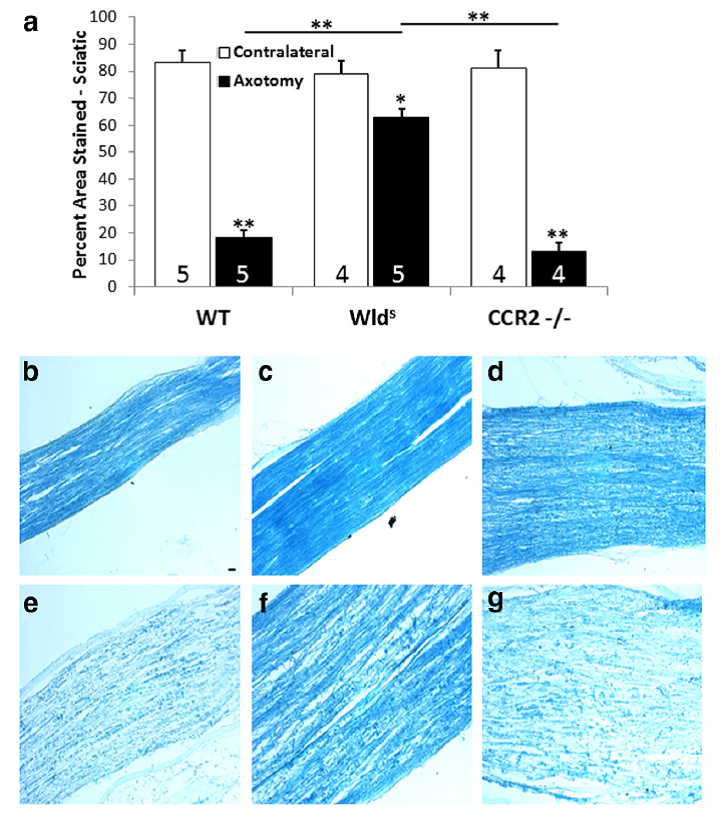
It has long been known that macrophages play a role in neural regeneration after axotomy; however, this influence has been viewed as occurring solely via an effect on Wallerian degeneration in the distal nerve segment. Some years ago, we discovered that, in addition to accumulating in the distal nerve, macrophages also accumulate around axotomized neuronal cell bodies. We have examined macrophage infiltration into DRGs and SCGs in two mutant strains of mice: the slow Wallerian degeneration mouse (Wlds) and a knockout for the receptor CCR2, the receptor on which the macrophage chemokine CCL2 acts. In both mouse strains, macrophage infiltration into the DRG was abolished and macrophage infiltration into the SCG was diminished. We then demonstrated that these macrophages within peripheral ganglia play an important role in peripheral nerve regeneration by activating neurite out-growth in response to a conditioning lesion (Fig. 12).
Fig. 12. Neurite outgrowth from explants of adult L5 DRGs ipsilateral (black bars) and contralateral (white bars) to a unilateral sciatic nerve transection 7 days previously in wild type (WT) and CCR2-/- mice. Growth of the 20 longest neurites was measured at 24 h (left-hand histogram) and 48 h (right-hand histograms) after placement in culture on a growth-permissive substrate. A conditioning lesion effect was seen in the contralateral but not the ipsilateral ganglia.
Another quite unexpected finding from these experiments came when we looked at Wallerian degeneration in the two mouse strains 7 days after unilateral transection using luxol fast blue staining. As expected the clearance of myelin in the Wlds mice was severely impeded; however, quite dramatically the results for the CCR2-/- mice were indistinguishable from controls, in spite of the fact that we found no infiltration of macrophages into the sciatic nerve in these animals (Fig. 13). These results raise the question of what cell type is the predominant phagocyte in the absence of infiltrating macrophage. Current work in the lab indicates an important role for neutrophils.
Fig. 13. (a). Quantitation of the myelin in the distal sciatic nerve after unilateral transection 7 days earlier and in the contralateral nerve. The micrographs represent sections from the ipsilateral (e– g) and contralateral (b– d) nerves from WT, Wlds, and CCR2-/-mice. *p<0.05, **p<0.001. Scale bar, 20 µm.
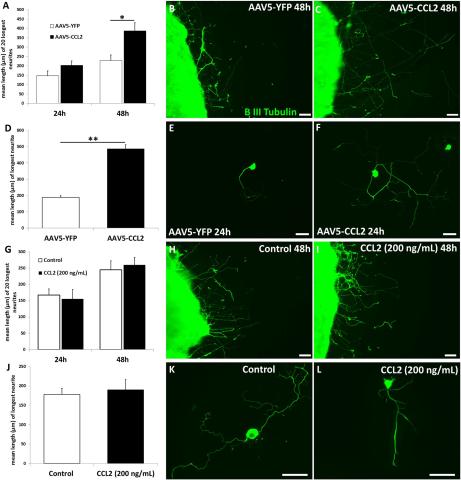
We next examined whether over-expression of the macrophage chemokine CCL2 stimulated neurite outgrowth. Control (uninjured) mice were injected intrathecally with an adeno-associated virus coding for CCL2. The virus infected neurons in the dorsal root ganglia and caused them to express CCL2. As a consequence, macrophages invaded the ganglia in spite of the fact that they had not been injured.
Fig. 14. Wild-type mice were injected intrathecally with AAV5-CCL2 or a control virus coding for YFP. Three weeks later when CCL2 expression and macrophage accumulation were at their maximum, DRGs were removed and placed in organ culture (A-C) or dissociated cell culture(D-F). Neurite outgrowth was enhanced by neurons from AAC5-CCL2 treated mice. The addition of recombinant CCL2 directly to control neurons produced no effect on neurite outgrowth (G-L)
Three weeks later when the ganglia were placed in organ culture or dissociated cell culture, neurite outgrowth was enhanced (Fig. 14). When the same protocol was performed in CCR2 knockout animals, no effect on neurite outgrowth occurred. The addition of CCL2 recombinant protein to control cultures also produced no effect on neurite outgrowth (Fig. 14), suggesting that the viral stimulation of neurite outgrowth depended on macrophage accumulation in the ganglia.
Publications (Since 2002)
- Talsma AD, Niemi JP, Pachter JS, Zigmond RE. The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration., J Neuroinflammation, (2022) In press.
- Niemi JP, DeFrancesco-Oranburg T, Cox A, Lindborg JA, Echevarria FD, McCluskey J, Simmons DD, Zigmond RE. The conditioning lesion response in dorsal root ganglion neurons is inhibited in oncomodulin knock-out mice, eNeuro, (2022) 9(1).
- Zigmond RE and Echevarria FD. Review: Macrophage biology in the peripheral nervous system after injury, Progress in Neurobiology, (2019) 173:102-121.
- Lindborg JA, Niemi JP, Howarth MA, Liu KW, Moore CZ, Mahajan D, Zigmond RE (2018).
Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation 15. - Lindborg JA, Mack M, Zigmond RE. (2017) Neutrophils are critical for myelin removal in peripheral nerve injury model of Wallerian degeneration. J Neurosci 37: 10258-10277.
- Niemi JP, Filous AR, DeFrancesco A, Lindborg JA, Malhotra NA, Wilson GN, Zhou B, Crish SD, Zigmond RE. (2017) Injury-induced gp130 cytokine signaling in peripheral ganglia is reduced in diabetes mellitus. Exp Neurol 296: 1-15.
- Niemi JP, DeFrancesco-Lisowitz A, Cregg JM, Howarth M, Zigmond RE. (2016) Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp Neurol 275: 25-37.
- Cohen MR, Johnson WM, Pilat JM, Kiselar J, DeFrancesco-Lisowitz A, Zigmond RE, Moiseenkova-Bell VY (2015) Nerve growth factor regulates transient receptor potential vanilloid 2 via extracellular signal-regulated kinase signaling to enhance neurite outgrowth in developing neurons. Mol Cell Biol 35:4238-4252.
- DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE (2015) Neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302:174-203.
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE (2013) A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve renegration. J Neurosci 41:16236-16248.
- Lingappa JR, Zigmond RE (2013) Limited recovery of pineal function after regeneration of preganglionic sympathetic axon: Evidence for loss of ganglionic synaptic specificity. J Neurosci 33:4867-4874.
- Pellegrino MJ, Parris DC, Zigmond RE, Habecker BA (2011) Cytokines inhibit norepinephrine transporter expression by decreasing Hand2. Mol Cell Neurosci 46:671-680.
- Hyatt Sachs H, Rohrer H, Zigmond RE (2010) The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp Neurol 223:516-22.
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE (2009) The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol 69:392-400.
- Hyatt Sachs H, Schreiber RC, Shoemaker SE, Sabe A, Reed E, Zigmond RE (2007) Activating transcription factor 3 induction in sympathetic neurons after axotomy: response to decreased neurotrophin availability. Neuroscience 150:887-97.
- Sachs HH, Wynick D, Zigmond RE (2007) Galanin plays a role in the conditioning lesion effect in sensory neurons. Neuroreport 18:1729-33.
- Zigmond RE, Vaccariello SA (2007) Activating transcription factor 3 immunoreactivity identifies small populations of axotomized neurons in rat cervical sympathetic ganglia after transection of the preganglionic cervical sympathetic trunk. Brain Res 1159:119-23.
- Shoemaker SE, Sachs HH, Vacariello SA, Zigmond RE (2006) Reduction in nerve growth factor availability leads to a conditioning lesion-like effect in sympathetic neurons. J Neurobiol 66:1322-1337.
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE (2005) A conditioning lesion enhances sympathetic neurite outgrowth. Exp Neurol 194:432-443.
- Schreiber RC, Boeshore KL, Laube G, Veh RW, Zigmond RE (2004) Polyamines increase in sympathetic neurons and non-neuronal cells after axotomy and enhance neurite outgrowth in nerve growth factor-primed PC12 cells. Neuroscience 128:741-749.
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE (2004) Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol 59:216-235.
- Nielsen KM, Chaverra M, Hapner SJ, Nelson BR, Todd V, Zigmond RE, Lefcort F (2004) PACAP promotes sensory neuron differentiation: blockade by neurotrophic factors. Mol Cell Neurosci 25:629-641.
- Schreiber RC, Vaccariello SA, Boeshore K, Shadiack AM, Zigmond RE (2002) A comparison of the changes in the non-neuronal cell populations of the superior cervical ganglia following decentralization and axotomy. J Neurobiol 53:68-79.
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME (2002) Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295:491-495.
Graduate Students' First Author Publications (Since 1992)
- Talsma AD, Niemi JP, Pachter JS, Zigmond RE. The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration. J Neuroinflammation. 2022 Jul 12;19(1):179. doi: 10.1186/s12974-022-02497-9.
- Lindborg JA, Niemi JP, Howarth MA, Liu KW, Moore CZ, Mahajan D, Zigmond RE. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation. 2018 Jun 26;15(1):192. doi: 10.1186/s12974-018-1222-5.
- Lindborg JA, Mack M, Zigmond RE. (2017) Neutrophils are critical for myelin removal in peripheral nerve injury model of Wallerian degeneration. J Neurosci 37(43): 10258-10277. PMID: 28912156
- Niemi JP, Filous AR, DeFrancesco A, Lindborg JA, Malhotra NA, Wilson GN, Zhou B, Crish SD, Zigmond RE. (2017) Injury-induced gp130 cytokine signaling in peripheral ganglia is reduced in diabetes mellitus. Exp Neurol 296: 1-15. PMID: 28645526
- Niemi JP, DeFrancesco-Lisowitz A, Cregg JM, Howarth M, Zigmond RE. (2016) Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp Neurol 275: 25-37.
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE (2013) A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve renegration. J Neurosci 41:16236-16248.
- Shoemaker SE, Sachs HH, Vacariello SA, Zigmond RE (2006) Reduction in nerve growth factor availability leads to a conditioning lesion-like effect in sympathetic neurons. J Neurobiol 66:1322-1337.
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE (2005) A conditioning lesion enhances sympathetic neurite outgrowth. Exp Neurol 194:432-443.
- Zhou Y, Deneris E, Zigmond RE (2001) Nicotinic acetylcholine receptor subunit proteins alpha7 and beta4 decrease in the superior cervical ganglion after axotomy. J Neurobiol 46:178-192.
- Bibevski S, Zhou Y, McIntosh JM, Zigmond RE, Dunlap ME (2000) Functional nicotinic acetylcholine receptors that mediate ganglionic transmission in cardiac parasympathetic neurons. J Neurosci 20:5076-5082.
- Ip NY, Zigmond RE (2000) Synergistic effects of muscarinic agonists and secretin or vasoactive intestinal peptide on the regulation of tyrosine hydroxylase activity in sympathetic neurons. J Neurobiol 42:14-21.
- Boeshore KL, Luckey CN, Zigmond RE, Large TH (1999) TrkB isoforms with distinct neurotrophin specificities are expressed in predominantly nonoverlapping populations of avian dorsal root ganglion neurons. J Neurosci 19:4739-4747.
- Mohney RP, Zigmond RE (1999) Galanin expression is decreased by cAMP-elevating agents in cultured sympathetic ganglia. Neuroreport 10:1221-1224.
- Mohney RP, Zigmond RE (1998) Vasoactive intestinal peptide enhances its own expression in sympathetic neurons after injury. J Neurosci 18:5285-5293.
- Zhou Y, Deneris E, Zigmond RE (1998) Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J Neurobiol 34:164-178.
- Sun Y, Zigmond RE (1996) Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci 8:2213-2220.
- Sun Y, Zigmond RE (1996) Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem 67:1751-1760.
- Sun Y, Landis SC, Zigmond RE (1996) Signals triggering the induction of leukemia inhibitory factor in sympathetic superior cervical ganglia and their nerve trunks after axonal injury. Mol Cell Neurosci 7:152-163.
- Sun Y, Rao MS, Zigmond RE, Landis SC (1994) Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol 25:415-430.
- Mohney RP, Siegel RE, Zigmond RE (1994) Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol 25:108-118.
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC (1993) Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron 11:1175-1185.
- Rao MS, Sun Y, Vaidyanathan U, Landis SC, Zigmond RE (1993) Regulation of substance P is similar to that of vasoactive intestinal peptide after axotomy or explanation of the rat superior cervical ganglion. J Neurobiol 24:571-580.
- Piszczkiewicz S, Zigmond RE (1992) Is the vasoactive intestinal peptide-like immunoreactivity in the rat pineal gland present in fibers originating in the superior cervical ganglion? Brain Res 598:327-331.
- Sun Y, Rao MS, Landis SC, Zigmond RE (1992) Depolarization increases vasoactive intestinal peptide- and substance P-like immunoreactivities in cultured neonatal and adult sympathetic neurons. J Neurosci 12:3717-3728.
- Balog BM, Sonti A, Zigmond RE. Neutrophil biology in injuries and diseases of the central and peripheral nervous systems. Prog Neurobiol. 2023 Jun 23;228:102488.
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE (2004) Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol 59:216-235.
- Shadiack AM, Sun Y, Zigmond RE (2001) Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurobiol 21:363-371.
- Ip NY, Zigmond RE (2000) Synergistic effects of muscarinic agonists and secretin or vasoactive intestinal peptide on the regulation of tyrosine hydroxylase activity in sympathetic neurons. J Neurobiol 42:14-21.
- Rittenhouse AR, Zigmond RE (1999) Role of N- and L-type calcium channels in depolarization-induced activation of tyrosine hydroxylase and release of norepinephrine by sympathetic cell bodies and nerve terminals. J Neurobiol 40:137-148.
- Nagamoto-Combs K, Vaccariello SA, Zigmond RE (1999) The levels of leukemia inhibitory factor mRNA in a Schwann cell line are regulated by multiple second messenger pathways. J Neurochem 72:1871-1881.
- Shadiack AM, Zigmond RE (1998) Galanin induced in sympathetic neurons after axotomy is anterogradely transported toward regenerating nerve endings. Neuropeptides 32:257-264.
- Shadiack AM, Vaccariello SA, Sun Y, Zigmond RE (1998) Nerve growth factor inhibits sympathetic neurons' response to an injury cytokine. Proc Natl Acad Sci U S A 95:7727-7730.
- Shadiack AM, Vaccariello SA, Zigmond RE (1995) Galanin expression in sympathetic ganglia after partial axotomy is highly localized to those neurons that are axotomized. Neuroscience 65:1119-1127.
- Schulz DW, Kuchel GA, Zigmond RE (1993) Decline in response to nicotine in aged rat striatum: correlation with a decrease in a subpopulation of nicotinic receptors. J Neurochem 61:2225-2232.
- McKeon TW, Zigmond RE (1993) Vasoactive intestinal peptide and secretin produce long-term increases in tyrosine hydroxylase activity in the rat superior cervical ganglion. Brain Res 607:345-348
Richard Zigmond Lab Members
Margaret Pinkevitch
Research Assistant
Brian Balog
Postdoc
Jon Niemi
Assistant Professor
Undergraduates
- TJ Disabato
- Anish Sonti
- Marius Patru
- Faye Hashim
Graduate Students' Job Placement
| Ph.D. Student | Present Position |
|---|---|
| Chauncey W. Bowers, Ph.D. |
Musician Songwriter CD available at chaunceybowers.com |
| Nancy Ip, Ph.D. |
President and Morningside Professor of Life Science Hong Kong University of Science and Technology Kowloon, Hong Kong |
|
Jaisri R. Lingappa, M.D., Ph.D. |
Retired - Previously Professor of Neurology Adjunct Professor - Department of Medicine Adjunct Professor - Department of Microbiology University of Washington UW Box 358116 750 Republican St, E583 Seattle, WA 98109 |
|
Michael Schwarzschild, M.D., Ph.D. |
Professor of Neurology Harvard Medical School MassGeneral Institute for Neurodegenerative Disease Massachusetts General Hospital Room No.3002, 114 16th Street Charlestown MA 02129 |
|
Yi Sun, Ph.D.
|
Professor Department of Psychiatry and Biobehavioral Science UCLA Medical School Los Angeles, CA 90095 - 7332 Professor and Director Translational Stem Cell Research Center Tongji University School of Medicine Shanghai 200065, China |
| Robert P. Mohney, Ph.D. |
Head of Clinical and Translational Science Owlstone Medical Research Triangle |
| Yuefang Zhou, Ph.D. |
Staff Scientist Washington University School of Medicine Department of Ophthalmology and Visual Sciences 660 S. Euclid Avenue St. Louis, MO 63110 |
| Sarah E Shoemaker, Ph.D. |
Research Mentorship Coordinator NC School of Science and Mathematics Durham, NC |
| Jon P. Niemi |
Assistant Professor Department of Neuroscience Case Western Reserve University Cleveland, OH 44106 |
| Jane A. Lindborg |
Study Director and Research Scientist Lovelace Biomedical Research Institute Albuquerque, NM |
| Aaron Talsma |
Medical Student Case Western Reserve University Cleveland, OH 44106 |
Postdoc's Job Placement
| Postdoc / R.A. | Present Position |
|---|---|
| Alcmene Chalazonitis, Ph.D. |
Retired: Formerly Senior Research Scientists Department of Pathology and Cell Biology Columbia University CPS 630 W.168th Street New York NY 10032 |
| Vincent A. Chiappinelli, Ph.D. |
Retired: Formerly Chair of Pharmacology and Physiology and Associate Dean George Washington University School of Medicine and Health Sciences 2300 | Street NW Washington DC 20037 |
| Ralph H. Loring, Ph.D. |
Associate Professor Department of Pharmaceutical Science Northeastern University 211 Mugar Hall Boston MA 02215 |
| Ann R. Rittenhouse, Ph.D. |
Associate Professor Dept. of Microbiology and Physiological Science University of Massachusetts Medical Center 368 Plantation Street Worcester MA 01605 |
| Catherine A. Sasek, Ph.D. |
Health Scientist Administrator National Institute on Drug Abuse 6001 Executive Blvd Rockville MD 20852 |
| George A. Kuchel, MD, FRCPC |
Professor of Medicine Citicorp Chair in Geriatrics & Gerontology Director, UConn Center on Aging Chief, Division of Geriatric Medicine University of Connecticut Health Center 263 Farmington Avenue, MC-5215 Farmington, CT 06030-5215 |
| David W. Schulz, Ph.D. |
Recently retired as Senior Director - Worldwide Business Development Pfizer Inc. 445 Eastern Point Rd. Groton CT 06340 |
| Annette M. Shadiack, Ph.D. |
Senior Director Senior Biological Subject Matter Expert Tunnell Government Services Washington, D.C |
| Kumi NagomotoCombs, Ph.D. | Assistant Professor Department of Anatomy and Cell Biology University of North Dakota School of Medicine and Health Science Grand Forks, ND 58203-281 |
| Kristen Boeshore, Ph.D. |
Associate Professor Biology Department Lebanon Valley College 101 N. College Ave. Annville, PA 17003 |
| Angela Filous, Ph.D. | Post-doctoral Fellow Department of Neurology Ohio State University 460 West 12th Avenue Columbus, OH 43210 |
| Franklin D. Echevarria, Ph.D. |
Research Site Manager Baptist Health Lexington Richmond, KY |
| Jon P. Niemi, Ph.D. |
Assistant Professor Department of Neuroscience Case Western Reserve University Cleveland, OH 44106 |
Medical Students' Job Placement
| Medical Student | Present Position |
|---|---|
| Daniel Mandell |
Cardiac Anesthesia Attending Department of Anesthesiology University of Pittsburgh Medical Center Pittsburgh, PA 15213 |
| Elliot Schwartz |
Anesthesiologist Cedar - Sinai Medical Center Los Angeles, CS |
| Amy Wax |
Professor of Law University of Pennsylvania Philadelphia, PA 19104 |
PREP Students' Job Placement
| PREP Students | Present Position |
|---|---|
| Lilinete Polsunas |
Resident Combined Pediatrics/Anesthesiology University of Pittsburgh Medical Center Pittsburgh, PA 15213 |
| Owen Shelton |
Entrepreneurial Fellow Chicago Biomedical Consortium Northwestern University Evanston, IL |
Undergraduates' Job Placement
| Undergraduate Student | Present Position |
|---|---|
| Gabrielle Leblanc, Ph.D |
Grant Consultant and Instructor QB3 UC Berkely 1700 4th St San Francisco, CA 94158 |
| Thomas Rando, MD, Ph.D |
Professor Department of Neurology and Neuroscience Stanford University School of Medicine Stanford, CA 94305-5253 |
| Usha Stiefel, MD |
Associate Professor of Medicine Chief, Infectious Diseases Section (LSCDVAMC) Case Western Reserve University Cleveland, OH 44106 |
| Caitlin Sedwick, Ph.D |
Freelance Science Writer csedwick@gmail.com |
| Christine M. LaPash Daniels, Ph.D |
Formerly Research Associate Waisman Cancer University of Wisconsin-Madison 1500 Highland Ave. Rm 713A Madison WI 53705 |
| Kevin Liu |
Medical Student University of Virginia Charlottesville, VA |
| Nisha Malhotra |
Medical Student at CWRU Case Western Reserve University Cleveland, OH 44106 |
| Mihika Thapliyal |
Medical Student Lerner College of Medicine Cleveland, OH |
| Sanika Paranjape |
Graduate Student Cognitive Neuroscience Program Department of Psychology George Washington University Washington, D.C |
| Deepti Mahajan |
Medical Student SUNY Downstate New York, NY |
| Krystal Scott | On Gap Year |
| Emad Pervez |
Medical Student Midwestern University Downers Grove, IL |
| Sahaj Bhambra | Current Student |
| Faye Hashim | Current Student |
| High School Student | Present Position |
|---|---|
| Rikki Rabinovich, PhD |
Postdoctoral Fellow University of Utah Neurosurgery Department |
| Madeline Howarth |
MD/PhD Student The Ohio State University Columbus, OH |
| Kathy Wong |
Undergraduate Student Columbia University New York, NY |
Research Assistants' Job Placement
| Research Assistant | Present Position |
|---|---|
| Claire Baldwin |
Retired Freelance Science Writer Belchertown, MA 01007 |
| Peter Rice PharmD, Ph.D |
Professor Department of Clinical Pharmacy University of Colorado Denver, CO |
| Lisa Dahm Ph.D |
Director, Clinical Informatics Director, ICTS Center for Biomedical Informatics UC Irvine Health Information Services University of California, Irvine |
| Rebecca Schreiber |
Papercut Artist Paper Confections Design |
| Stacey Koeth |
Business Development Manager AmericanBIO, Inc. 15 Erie Drive Natick, MA 01760 |
| Hilary Sachs |
Research Editing Services hhsachslst@gmail.com |
| Talia DeFrancesco Oranburg, PhD |
Variant Curation Scientist Ariel Precision Medicine Pittsburgh, PA |
|
Jeong Seo |
Research Scientist BrainXell Madison, WI |