Investigators at the Center for Imaging Research aim to transform diagnostics and therapeutics
The vision of the Case Western Reserve University Center for Imaging Research (CCIR) is to lead the next generation of transformative imaging research that will radically improve disease prevention, diagnosis and therapy. One of the diseases targeted by CCIR’s multidisciplinary investigators is prostate cancer.
“Prostate cancer is an insidious disease – the most prevalent cancer among men in the United States and the No. 2 killer in men,” says James P. Basilion, a CCIR researcher and professor of biomedical engineering and radiology. “While the diagnostic and therapeutic approach to prostate cancer has been revolutionized, a lot of work still needs to be done.”
Basilion and several of his colleagues at CCIR are developing new technologies and tools to advance the field with modalities including magnetic resonance imaging, magnetic resonance fingerprinting, ultrasound and others. Here are four of their cutting-edge projects focused on prostate cancer:
Preventing a Toxic Side Effect of RLT
Traditional radiation therapy is delivered via external beam irradiation, which travels through the body on route to the tumor. The development of targeted radioligand therapy (RLT) – which attaches a radionuclide to a ligand that binds to prostate-specific membrane antigen (PSMA) – delivers treatment directly to the tumor and spares radiation to healthy tissues and organs.
“RLT is currently enjoying great commercial success for treatment of prostate cancer,” says Zhenghong Lee, a professor of radiology and biomedical engineering. “However, an unexpected toxic side effect quickly surfaced as patients complained about having severe dry mouth, making it difficult to eat, swallow and speak.”
There are no remedies for targeted RLT-induced xerostomia. Lee and his colleagues, including Basilion, are developing an approach to prevent the side effect.
“We have discovered that a small molecule inhibitor – a shield – can protect the salivary glands and prevent dry mouth during RLT,” says Lee. The aim of the research is threefold:
- To optimize the salivary blocking effect based on biodistribution and radiation dosimetry.
- To examine toxic profiles of the shield to prepare it for clinical translation.
- To further test the improvement in alpha/beta particle therapies with salivary protection.
The team has completed preclinical and therapy experiments, as well as a two-species toxicology study for the inhibitor showing a favorable safety profile.
“Currently, RLT is a last resort when patients fail all available treatment options,” says Lee. “If proven clinically successful, our shield could be used so that many existing targeted RLTs could move forward for more aggressive treatment as a frontline or second-line treatment.”
Improving Image-Guided Biopsies
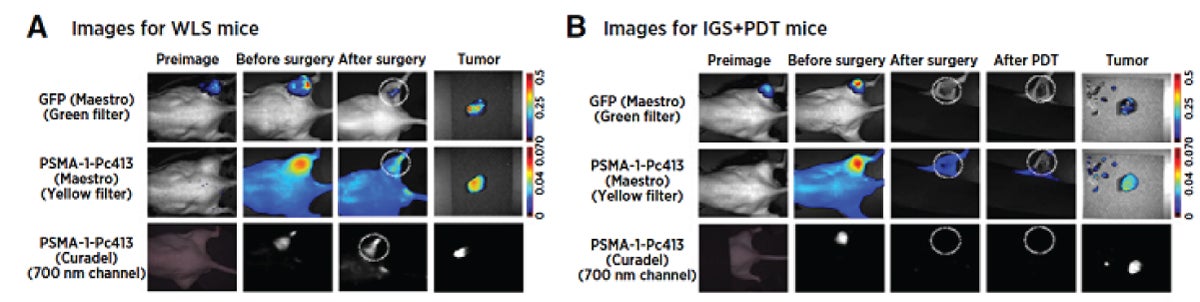
Xinning Wang, a research associate professor in the Department of Biomedical Engineering and a member of Basilion’s lab, has developed an improved PSMA-binding molecule for use as a targeted theranostic agent for fluorescence-guided surgery and photodynamic therapy. “We designed our ligand – PSMA-1 – so that it has better selectivity, better specificity and less off-target binding,” says Basilion.
Basilion is now using PSMA-1 to “begin to approach problems that others haven’t approached,” he says. Being a part of the multidisciplinary imaging research community at CCIR expedites that work. One of his latest
collaborations is with Agata Exner, faculty director of CCIR, the Henry Willson Payne Professor and vice-chair for basic research in radiology, and a professor of biomedical engineering.
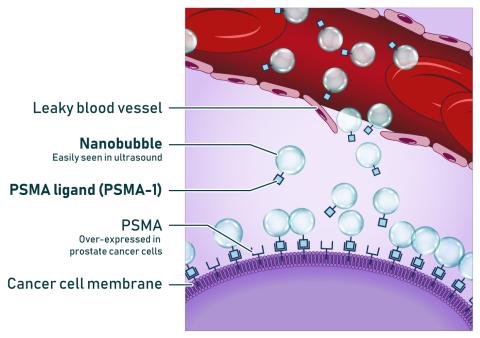
Exner’s research centers around nanobubbles – nanoparticles with gas at their core. By stimulating the nanobubbles with sound waves, she hopes to improve cancer detection and targeted therapy. Joining PSMA-1 with nanobubble contrast agents offers promise for one of Exner’s focus areas – ultrasound imaging of prostate cancer.
Some of the limitations of traditional ultrasound-guided prostate biopsies include poor spatial resolution and limited sensitivity, which can lead to challenges in identifying and characterizing tumors.
“We found that combining the PSMA-1 ligand with nanobubbles, which can be visualized with ultrasound imaging, we can bind to cancer within the prostate gland,” says Basilion. “We can get a signal-to-noise background ratio that makes it possible to visualize the tumor over the normal prostate tissue, allowing for better image-guided biopsies.”
The researchers launched the startup company Visano Theranostics to further develop the theranostic agent.
Reducing Unnecessary Biopsies
Yong Chen’s research also focuses on prostate biopsies, specifically reducing unnecessary procedures. Approximately 30% of patients with a negative MRI for prostate cancer end up getting a biopsy due to suboptimal negative predictive values with current MRI techniques. This leads to unnecessary biopsies and post-procedure complications.
Chen’s team is developing a fast diffusion magnetic resonance fingerprinting (MRF) technique. “Unlike conventional imaging methods which provide qualitative tissue contrasts, MRF can generate precise tissue property measurements, allowing for more comprehensive tissue and disease characterization,” says Chen, an assistant professor of radiology.
His team has pioneered an MRF technique that enables accurate quantification of T1 and T2 relaxation times in prostatic tissues. “Our preliminary data suggests that this approach could help reduce unnecessary biopsies by approximately 60% in patients with a negative MRI,” says Chen.
Water diffusion is another critical tissue property that could further improve prostate cancer detection and grading. As part of a 5-year, $3.1 million award from the National Cancer Institute, Chen and his CCIR colleague, the project’s Principal Investigator Leonardo Kayat Bittencourt, MD, PhD, vice chair of innovation in the Department of Radiology at UH Cleveland Medical Center, along with Dan Ma, associate professor of neurosurgery and biomedical engineering at Duke University, will optimize the prostate MRF technique for T1, T2 and diffusion quantification.
“The ultimate goal of this project is to develop a multivariable predictive model that integrates MRF-derived quantitative values with patient clinical information,” says Chen. “Based on this tool, clinicians can more accurately and objectively rule out prostate cancer, reduce unnecessary biopsies, minimize potential complications and lower healthcare costs for patients with a negative MRI.”
Developing Minimally Invasive Surgical Procedures
Researchers at Case Western Reserve University and Vanderbilt University are pioneering a new approach to prostate cancer surgery by combining low-field MRI techniques with a surgical robot. The goal is to replace radical prostatectomy with minimally invasive focal resection of prostate cancer.
“Targeted removal of localized prostate lesions could alleviate complications associated with total prostate gland removal, but it is challenging because cancerous tissue can appear identical to healthy tissue in endoscopic images, making accurate surgery difficult,” says William Grissom, the Medtronic Professor of Biomedical Discovery and Innovation at Case Western Reserve School of Medicine and a professor of biomedical engineering. Grissom and Robert Webster, the Richard A. Schroeder Professor of Mechanical Engineering at Vanderbilt, are leading the collaborative project, which received a five-year, $3.7 million grant from the National Institutes of Health.
The innovative surgical approach hinges on two components – a robot from Virtuoso Signals Inc., a startup founded by Webster, and a low-field MRI scanner from Promaxo Inc. Grissom and Webster will adapt the robotic device, which was originally built for holmium laser enucleation of the prostate (HoLEP), a total prostate resection via a transurethral procedure.
“The robot is currently only suitable for removing the whole prostate because it can’t see lesions,” says Grissom. “In order to excise only the cancerous part of the prostate, they need some additional information.” That’s where the low-field MRI scanner comes into play.
The team will combine high-field magnetic resonance images obtained prior to the procedure with real-time images gathered continuously from the low-field MRI scanner during the procedure.
“We know that low-field MRI doesn’t have enough contrast to show the lesions clearly on their own,” says Grissom. “But they have similar contrast to the high-field images, so we should still be able to see landmarks and do a better job of registering those two images together than going across modalities, such as ultrasound-MRI fusion.” The adapted robot will carefully guide two needle-sized manipulators through a standard transurethal endoscope directly to the lesions identified on the high-field images.
“This will make the focal resection process much easier and more accurate for the surgeon,” says Grissom. “The system will enable personalized resections for patients who today have no alternative to radical prostatectomy.”