Every two seconds someone in the U.S. needs a blood transfusion, according to the American Red Cross.
“Blood transfusion is a highly critical, life-saving clinical procedure for a variety of patients, including people bleeding out from severe injury or undergoing surgery, as well as those whose native blood doesn’t function adequately due to disease or medication effects,”
says Anirban Sen Gupta, the Wallace R. Persons Endowed Professor of Engineering in Case Western Reserve University’s Department of Biomedical Engineering. “The transfusion logistics become highly problematic when you realize that blood availability is completely donor driven and there is often a paucity of donors, especially in scenarios such as the recent COVID pandemic.”
Case Western Reserve University is one of several universities and industry partners working on a solution as part of a four-year, $46.4 million project funded by the Defense Advanced Research Projects Agency (DARPA). The project aims to develop a bioartificial whole blood surrogate.
The blood surrogate will combine, evaluate and optimize three biosynthetic components that mimic the function of platelets, red blood cells and plasma respectively. An additional focus of the project is to establish that the optimized artificial blood can be freeze-dried and stored as a powder under various environmental conditions so that it can then be reconstituted on demand using saline.
“There’s been a lot of research on artificial blood for decades, ramping up during the AIDS pandemic in the 1980s. But most of the work in the past focused on a single component – red blood cells,” says Sen Gupta. “This is indeed the world’s first attempt to try to make artificial whole blood that includes mimicking other critical components, such as platelets and plasma.”
Tackling Challenges in Blood Donation and Transfusion
The researchers hope that creating a viable blood surrogate will overcome several challenges associated with donor-derived blood usage. First is availability: Blood product need and utilization often outpace blood donations. Logistics and portability present another hurdle.
“Even if you get ample donations, you have to type match blood, bank it and get it where it needs to be,” says Sen Gupta. While most transfusions occur within hospitals, in some scenarios – such as traumatic bleeding due to injury – the earlier you can transfuse the blood, the better the survival outcomes.
“Whether it’s a civilian car accident or a military battlefield, access to blood needs to be rapid. Ideally, the blood should be taken to the patient rather than waiting for the patient to come to the hospital,” says Sen Gupta.
Finally, blood has a fixed shelf life. Whole blood can be stored between 1 and 6 degrees Celsius for approximately a month. Frozen plasma is viable for several months, while cooled platelets have a shelf life of approximately two to three weeks.
“If you take into account the shortage in donation and limited shelf life, the challenges are amplified,” says Sen Gupta. “We just don’t have enough blood, and even if we do, we can’t stockpile it.”
The DARPA project strives to address all of these issues and develop a synthetic system that functions like biologic blood, can be manufactured at large scale, doesn’t require type matching and can be freeze-dried, stored and transported anywhere in the world.
“All of these are hard problems, and we are trying to solve them in parallel,” says Sen Gupta.
Partner Roles
The components being combined and tested in the whole blood surrogate include:
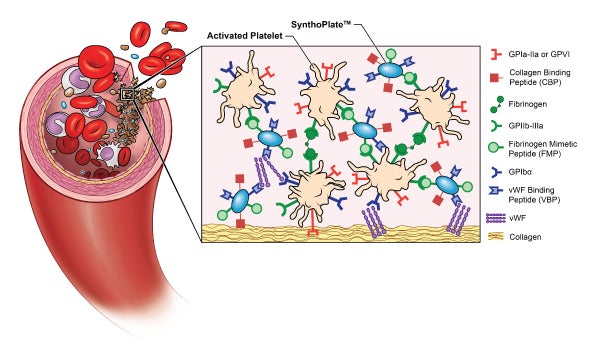
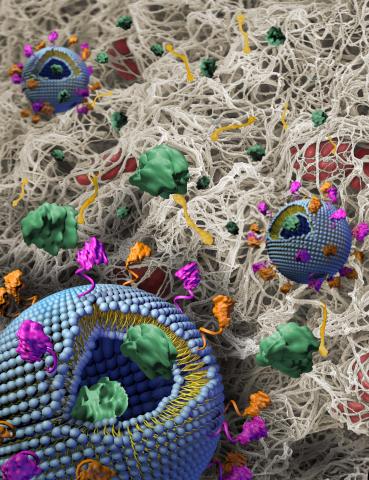
- SynthoPlate™, a synthetic hemostatic agent developed by Sen Gupta and licensed to Haima Therapeutics
- ErythroMer, a dried red blood cell substitute made by KaloCyte
- Freeze-dried plasma from Teleflex
While the individual components are important, it’s imperative that they work together to mimic the critical functions of real blood.
“In a reductionist view, we can boil down the function of blood to three main roles – oxygenation, hemostasis and hemodynamics,” says Sen Gupta. “Our role at CWRU is to integrate all of the artificial components and use a multitude of in vitro techniques and methods to evaluate and optimize hemostasis, which is our natural ability to form a clot to stop bleeding.”
Researchers at the University of Maryland are studying oxygenation, and a team at the University of California San Diego is examining hemodynamics. After the universities have agreed on an optimal formulation, it will be studied in vivo at the University of Pittsburgh, which is also responsible for blood banking and comparing the artificial whole blood to stored real blood.
The four-year DARPA project has set ambitious non-inferiority criteria: Each of the three domains – oxygenation, hemostasis and hemodynamics – must be 90% as effective as donor-derived stored blood used in transfusions. There are intermediate goals of 60% functionality at the end of year one, 70% at the end of year two and 80% at the end of year three. Case Western Reserve University has met the 60% non-inferiority margin for hemostasis within the first year, which ended in January 2024.
Analyzing Clot Formation
Now in the second year of the project, Sen Gupta’s laboratory continues to conduct performance analyses of the blood surrogate. Meanwhile, safety parameters are being studied in the laboratories of three co-investigators: Harihara Baskaran, chair of the Department of Chemical Engineering; Umut Gurkan, William J. Austin Professor of Engineering in the Department of Mechanical and Aerospace Engineering; and Pedram Mohseni, Goodrich Professor of Engineering Innovation and chair of the Department of Electrical, Computer and Systems Engineering.
The performance analyses examine various aspects of clot formation, including how soon the clotting begins, how fast the clot grows and whether the clot remains stable or breaks off.
“Ultimately, the artificial blood system is going to be in circulation in a human body, so it is important that it doesn’t cause any kind of harm – a negative immune response, unnecessary clotting or altering viscosity and flow velocity through the blood vessel,” says Sen Gupta. “All of these are safety parameter analyses that we are studying in parallel.”
Each week, the researchers perform multiple experiments to compare human donor-derived blood to engineered artificial blood. The experiments:
- Measure the kinetics, robustness and stability of clotting
- Image the cellular and molecular mechanisms of blood clotting in real time
- Assess the rheology of flowing blood in physiology, simulating microfluidic systems
- Investigate any possible harmful effects of the blood on endothelial cells and immune cells
Scaling Up and Deploying the Product
While universities are analyzing oxygenation, hemostasis and hemodynamics, industry partners are working on the logistics of producing synthetic whole blood at scale.
“We have to confirm that scale up doesn’t affect the function. It might work in one milligram, but what if it fails in one gram scale?” says Sen Gupta. “Also, what if a freshly made system works great, but when you freeze dry it and then reconstitute in saline it doesn’t work that well anymore?”
Sen Gupta and the other researchers communicate regularly with teams from Haima Therapeutics, KaloCyte and Teleflex and refine the blood surrogate formulations as they strive to create a functioning whole blood substitute. In addition, the Southwest Research Institute is spearheading efforts to scale up production and create sterilized packaging for rapid deployment.
The lifesaving potential of synthetic blood is vast. Soldiers could carry freeze-dried blood in their modular lightweight load carrying equipment (MOLLE) to treat injury on the battlefield. Ambulances could stock it for emergency medical personnel to give blood transfusions to people following car accidents, mass casualty events or other emergencies. Hospitals could stock artificial blood for on-demand use in surgeries, injuries, disease and bleeding disorders.
“All of the partners in the consortium are excited to be part of this historic effort,” says Sen Gupta. “This endeavor is the first to invest in integrating the oxygen-carrying function with clot formation and hemodynamics to create a product that is functionally close to whole blood.