Identifying key mediators of tissue regeneration downstream of 15-PGDH inhibition
Project Description:
I am currently a doctoral trainee in Dr. Sandy Markowitz’s lab. One focus of the Markowitz lab is to investigate how inhibitors of 15-prostaglandin dehydrogenase (15-PGDH) resolve inflammation and lead to tissue regeneration in ulcerative colitis. Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) involving inflammation of the superficial layer of the colon and rectum. To date, UC has no known preventive or curative treatments, and often involves lifelong treatment for patients. An emerging target for ulcerative colitis therapies focuses on mucosal healing, or regeneration of the epithelial layer of the colon and rectum, with evidence suggesting that mucosal healing resulted in improved long-term clinical outcomes such as reduced risk of relapse. The Markowitz laboratory previously showed that inhibition of 15-PGDH, which enzymatically degrades prostaglandin PGE2, results in increased levels of tissue PGE2 and sustained colonocyte proliferation, leading to near complete protection from ulceration in the colon of mice with dextran sulfate sodium (DSS)-induced colitis. However, the mechanisms by which 15-PGDH inhibition and accumulation of PGE2 lead to tissue regeneration are unclear.
For my thesis project, I will interrogate drug effects on cell populations of the colon mucosa using single-nuclei RNA-sequencing (snRNA-seq) from DSS-colitis mice treated with or without SW033291 (a 15-PGDH inhibitor). I propose to use snRNA-seq to specifically identify a network of early DSS-induced responses in colon stem cells that are reversed with SW033291. I further hypothesize that using snRNA-seq to interrogate in vivo effects of SW022391 will identify additional biologically relevant genes, pathways, and cell types that are necessary for tissue regeneration downstream of 15-PGDH inhibition. I will interrogate our top targets by confirming changes in mRNA and protein expression with SW033291 treatment in mouse DSS-colitis models using techniques such qPCR, western blot, and bioimage analysis of in situ hybridization- and immunofluorescence-stained tissue sections. I will then confirm the functional importance of our targets using knockout mouse models. These results will illuminate how a novel class of ulcerative colitis drugs works and accelerate the delivery of new therapeutics from bench-to-bedside.
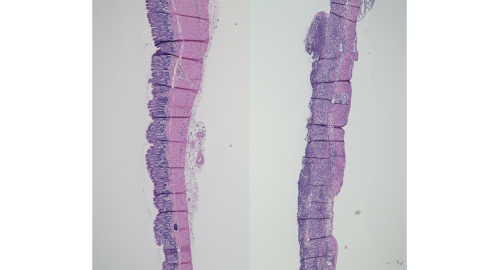
Figure: Formalin-fixed paraffin-embedded tissue sections prepared from the distal colons of naïve wild type mice (left) and DSS-exposed wild type mice (right) stained with hematoxylin and eosin show healthy colon crypts (left) versus complete loss of colon crypts (ulceration) induced by DSS-colitis (right).