Delineating IER3IP1 epistasis to understand its function and MEDS pathogenesis
Project Description:
I am currently a doctoral trainee in Dr. Ashleigh Schaffer's lab. One focus of the Schaffer lab is uncovering new mechanisms of rare, pediatric neurological disorders through stem cell technologies and genetic tools. I am interested in exploring secretory protein trafficking in neurological system. Neural progenitor cells (NPCs) differentiate into neurons with unique and specialized cellular architectures, including a cell body, axon, and extending dendrites. During neurogenesis, spatial and temporal regulation of secretory protein trafficking is critical, and efficient protein trafficking is paramount for maintaining neuronal function. Thus, defects in the trafficking process result in neurodevelopmental and neuropsychiatric disorders in patients. However, gaps in the understanding of secretory protein trafficking pathways in human neurons impose challenges for defining the molecular pathogenesis and identifying therapeutic targets of such neurodevelopmental disorders. For my project, I propose to employ a genome-wide epistasis screen to elucidate the neuronal secretory protein trafficking pathway in healthy and diseased states and identify targets of therapeutic interest.
To do so, I developed a novel screening platform using induced pluripotent stem cells (iPSCs) derived NPCs from patients with Microcephaly, Epilepsy and Diabetes Syndrome (MEDS; OMIM #614231), caused by a homozygous IER3IP1 p.L78P variant that severely impairs secretory protein trafficking. The role of IER3IP1 in secretory protein trafficking and the pathogenesis of MEDS remains elusive. However, previous literature and my preliminary data suggest that IER3IP1 is involved in ER-Golgi protein trafficking. For this project, I aim to perform a genetic epistasis screen with MEDS patient-derived NPCS and controls and identify novel genes that will have a robust effect on secretory protein trafficking in NPCs. The results will offer invaluable insights to deepening the mechanistic understanding of process of secretory protein trafficking during neurodevelopment and the pathophysiological function of IER3IP1 in MEDS.
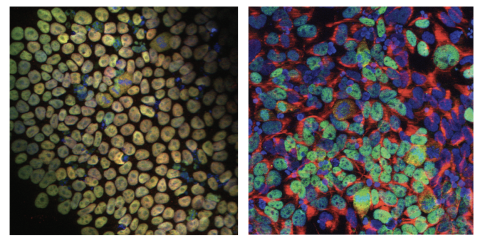
Immunofluorescence staining images of iPSCs and NPCs derived from the MEDS patient’s fibroblasts. iPSCs are stained for pluripotency markers (NANOG, SOX2), whereas NPCs are stained with progenitor cell markers (NESTIN, PAX6) along with DAPI staining.