Umut Gurkan, PhD, CTS Pilot Co-Lead
Ian Neeland, MD, FAHA, FACC, CTS Pilot Co-Lead
Amar Desai, PhD, CTS Pilot Co-Lead
Anna Thornton Matos, MPH, Administrative Lead, Research Pilot Program
Annual Pilot Technical Webinar, replay now available!
LOI submission due via InfoReady, October 20, 2025 at 11:59PM
Mid Dec 2025 LOI results announced
January 19, 2026 Full proposals due
April 3, 2026 Annual Pilot awards announced
May 31, 2026 Any applications with IRBs must have IRB approval IN PLACE
July 1, 2026 Annual Pilot project start date
June 30, 2027 Annual Pilot project end date
Annual Pilot Information
| Title: | Clinical and Translational Science Annual Pilot Award Program 2026 |
| Available Funds: | Up to 8 pilot awards at up to $50,000 each |
| Funding Period: | July 1, 2026 - June 30, 2027 |
| Letter of Intent Deadline: | CLOSED (10/20/25) |
| Funding Source: | NIH National Center for Advancing Translational Sciences |
Technical Webinar (September 15, 2025) Replay
The Clinical and Translational Pilot Award Program is funded through the NIH National Center for Advancing Translational Sciences (UM1TR004528), which defines translation as “the process of turning observations in the laboratory, clinic and community into interventions that improve the health of individuals and the public — from diagnostics and therapeutics to medical procedures and behavioral changes.” (https://ncats.nih.gov/translation)
The goals of the CTSC Annual Pilot Program are:
- To foster innovative new clinical and translational research that supports research development to address urgent questions or to develop preliminary data that can provide initial support to establish proof of concept and be used to launch new NIH or other externally-funded research proposals;
- To encourage interdisciplinary teams of investigators that span across CTSC partners to stimulate investigators from other areas to lend their expertise in research in clinical and translational science while developing collaborations or new research avenues;
- To support research collaborations with community partners and organizations; and
- To advance health in Northern Ohio through research
Projects must be feasible within the proposed 12-month timeframe, have high methodological and scientific quality, and answer important scientific questions. Up to (8) grants, will be awarded with budgets of up to $50,000 each in direct costs. All research activities must be completed by June 30, 2027.
Priority Areas:
Although applications are welcome in the broad domains of clinical and translational research, we are especially interested in applications covering the following priority areas for 2026 funding:
- Projects addressing clinical translational science; that is, disease agnostic projects that focus on the process of turning research results into real-world applications to improve health
- Innovative, multi-center clinical studies that integrate community and clinical partners to ensure health improvements for all
- Dissemination & Implementation of research evidence and innovation to clinical practice
- Projects that leverage Cosmos or TriNetX
- Projects addressing the health concerns and conditions of rural Ohio
- Innovative use of Artificial Intelligence (AI)
- Innovative Implementation Science methods that promote the uptake of research findings into routine healthcare in clinical or policy contexts
- Interventions at community and/or clinical practices to promote overall health
- Studies on methods to improve the clinical and translational research process
- Projects that focus on the following current topics: gene editing, microbiome, deep learning, robotics in healthcare, drug discovery, advances in medical imaging, improving the reproducibility and reliability of scientific findings
Applicants will first submit a Letter of Intent (LOI) to clearly enunciate the proposed activities that address one or more of these goals with a sustainable design for future application to community health. Applicants submitting a successful LOI will be invited to submit a full proposal.
Eligibility and Review Criteria
-
We encourage the Multiple Principal Investigator (MPI) model for these submissions. The PI(s) must be a Case Western Reserve University (CWRU) faculty member from CWRU, Cleveland Clinic, MetroHealth Medical Center, University Hospitals, or the Veterans Affairs Northeast Ohio Health Center and eligible to be a PI for a NIH grant. Co-I’s may include faculty at University of Toledo and NEOMED, community health leaders, trainees, and coordinators.
-
All applications must include 2 or more CTSC partner institutions or they will not be considered for funding.
-
All CWRU Schools may apply for this initiative.
-
Applications from new investigators are highly encouraged.
-
The ideal letter of intent will address a mid- to late-stage clinical research (T2/T3/T4; see Appendix 1) of community health with an articulated, quantitative and/or qualitative outcome result leading to a change in health using Translational Research or Translational Science approaches.
Examples which will be more favorably reviewed will include projects that:
- Demonstrate team building across biological and behavioral methodology and clinical health science
- Articulate the innovation for the applicant(s) and/or the co-investigator(s)
- Use community-engaged research principles to address equity in deployment of the outcomes
- Incremental impact, if any, of a novel strategy to improve health in less resourced communities
Budget Considerations (see Appendix 2)
Case Western Reserve University will serve as the fiscal entity through which CTSC and/or CTSC/Case Coulter Translational Research partnership funds for technology transfer will be distributed and administered. The amount of the pilot award can be lower, depending on the scope and type of the project, and proposals can identify opportunity for new supplement funds from additional institutional, departmental, or private funds.
Other budget information:
- Maximum of $5,000 in salary support for established investigators who do not currently have active NIH support as a PI or co-PI; established investigators with active NIH support as PI or co-PI are ineligible for salary support. There is no salary limit for early-stage investigators (early-stage is defined as "has not been a PI for a substantial NIH research award").
- Budget for supplies and investigative purposes must be well justified.
- No funds will be provided for administrative assistance, office equipment and supplies, computers, tuition, travel, purchasing and binding of periodicals and books, dues and membership fees in scientific societies, honoraria and travel expenses for visiting lecturers, recruiting and relocation expenses for faculty or staff, office and laboratory furniture, rental of office or laboratory space, per diem charges for hospital beds, non-medical or personnel services to patients, construction or building maintenance, or major alterations.
Number of PIs
One CWRU investigator will be the responsible PI and may only receive one award in the budget year. Additional collaborating investigators will be named. If MPIs are included, one of the PIs should be clearly designated as the contact PI and as such, will be the PI fiscal contact. If awarded an Annual Pilot, you are ineligible to receive another Annual Pilot for at least two years.
New Investigators
For the purpose of this RFA, a new investigator is defined as a faculty member who is not tenured and who has not been a faculty member at CWRU or any other institutions for more than six years in aggregate. The review panel can assign extra weight to a proposal from a new investigator to enhance opportunity for funding.
Trans-Disciplinary and Trans-Institutional Team Science
Including co-investigators with diverse skills from different schools, colleges or community organizations is required. A project where one of the investigators is simply providing access (to data, specimens, or patients) is less acceptable than one where there is knowledge or skill sets enhance the Annual Pilot and leads to building a Team for continuation of the theme.
Utilization of a CTSC Core Facility
If a CTSC Core is utilized, detailed documented consultation from the specific CTSC Core director or his/her designee for that CTSC Core is required. Justification for use of the CTSC core(s) must be included in the LOI. Applicants can apply for both an Annual Pilot ($50,000) and a Core Utilization Pilot ($10,000 from the PI home institution) if a CTSC Core is being used. The Core Utilization Award, however, is for 6 months.
More than one proposal per faculty member serving as a co-investigator may be submitted; however, if the LOIs are accepted, conflicts must be addressed in the best interests of the CTSC program goals.
Change of Institutions/Transfers
Recipients may not transfer these awards to another institution or to another individual. Reallocations in the approved budget require prior written approval before expenditure. Awardees are required to give 90 days’ notice of any change in Institution, and funds will be prorated when an applicant leaves the Institution.
Clinical and Translational Science Collaborative and Case-Coulter Translational Research Partnership Funding
The CTSC and CCTRP may provide joint funding to faculty for Annual Pilot Projects that will support inter-institutional, clinical and/or technological translational research in the City of Cleveland. CTSC/CCTRP projects are expected to have a Biomedical Engineering faculty member as an integral Co-Investigator. If you have questions about the CCTRP funding, please visit the Case-Coulter website.
Letter of Intent Submission Process
Investigators interested in submitting applications to the Annual Pilot Program have the option to first schedule a consultation with the CTSC Pilot Program faculty lead and Research Navigator prior to submitting a Letter of Intent (LOI) in InfoReady. To schedule a consultation, select “Research Navigation Services” in SPARCRequest.
Investigators submitting a proposal appropriate to the goals of the Annual Pilot Grant Program will be invited to submit a
full application (See Appendix 3).
The LOI is a short summary of the proposal (one page maximum) that must address the following:
- Project Title
- Names and Affiliations of PI, Co-PIs, Co-Investigators and Collaborators; ensure all Project Site(s) are included
- A brief, focused project summary:
- Question/Hypothesis of the study - This section should be about 1⁄4 of a page and should describe the overall health related goal addressed by the hypothesis/premise/scientific question. The one-year Pilot can be within a larger development plan but should stand alone as a milestone to the larger effort.
- Innovation/Translation - This section should be about 1⁄4 of a page and describe how your project fills a critical translational gap, and a discussion of anticipated results.
- Feasibility - This section should be about 1⁄4 of a page and describe the feasible elements of your one-year study; include potential roadblocks or concerns in this section.
- Project Milestones - This should describe the project plan in about 1⁄4 page. For the scope of this project we suggest no more than 3 temporal or decision-making milestones that can be achieved with the budget allowed (up to $50,000) and in the time frame selected (up to one year). Please include an estimate of how long each milestone will take, and why it is critical, to the proposal and any long-term plans.
- Statement of Translation - a statement as to why the project is innovative mid- to late-stage Translational Research or Translational Science (T2/T3/T4).
- Budget Summary - statement of how the award funds would be utilized and distributed
- Human Subjects - If the study requires IRB approval, include a statement outlining plans to obtain IRB approval by April 30, 2025, dates of submission and/or approval. (An already approved or nearly approved IRB will be favorably reviewed.)
- Intellectual Property - Has this project been submitted to or received Intellectual Property protection? If yes, do you have a plan to file an invention disclosure? If no, would you like help to learn more?
- Special Considerations - Statement whether this project is planned to have any foreign components, MOUs, or sub-awards.
Important Dates:
| September 15, 2025 | Annual Pilot Technical Webinar |
| October 20, 2025 | LOI submission due via InfoReady |
| Mid December | LOI results announced. Successful applicants will be invited to submit a full proposal that will include more details on the premise, goals, milestones, personnel, and detailed budget, along with the IRB submission status (revision or approval). |
| January 19, 2026 | Full proposals due |
| April 3, 2026 | Annual Pilot awards announced |
| May 31, 2026 | Any applications with IRBs must have IRB approval IN PLACE |
| July 1, 2026 | Annual Pilot project start date |
| June 30, 2027 | Annual Pilot project end date |
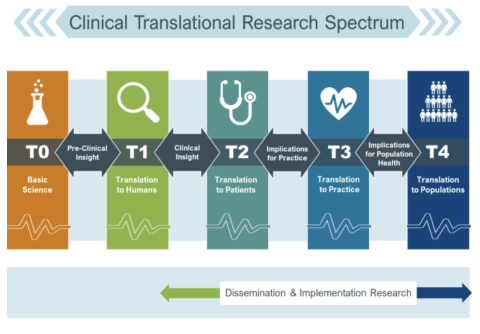
Appendix 1.
Translational Spectrum and Definitions
Source: https://research.musc.edu/resources/sctr/programs/disc
| Translation | The process of turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and communities – from diagnostics, preventions, and treatments to medical procedures and behavioral changes. |
| Translational Research | The endeavor to traverse a particular step of the translational process for a particular target or disease. |
| Translational Science | The field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process. Translational Science is disease agnostic. |
Basic Science (T0)*
The basic research stage of translation is research to understand the human condition and environment by studying the biological, social, and behavioral mechanisms that underlie health and disease. Methods include preclinical or animal studies, and association studies using large datasets.
Pre-Clinical Research (T1)*
The pre-clinical research stage of translation is the first stage of translation research which is the process of moving research findings from the lab to patients and communities. This involves pre-clinical studies, developing protocols for human clinical trials, and finding new methods of diagnosis, treatment, and prevention.
*Basic Science (T0) and Pre-Clinical Research (T1) are NOT the focus for the 2025 Annual Pilot Award. (Instead, we invite you to apply for a CTSC Voucher or Core Utilization Pilot for these types of projects)
The areas of funding for this RFA include:
Clinical Research (T2)
The clinical research stage of translation transfers findings from clinical studies or clinical trials to practice settings and communities, where the findings improve health. This stage includes translation to patients, including Phase 2 and 3 clinical trials, and controlled studies leading to clinical application and evidence-based guidelines.
Clinical Implementation (T3)
The clinical implementation stage of translation involves the adoption of interventions that have been demonstrated to be useful in a research environment into routine clinical care for the general population. This stage also includes implementation research to evaluate the results of clinical trials and to identify new clinical questions and gaps in care.
Public Health (T4)
In this stage of translation, researchers study health outcomes at the population level to determine the effects of diseases and efforts to prevent, diagnose and treat them. Findings help guide scientists working to assess the effects of current interventions and to develop new ones.
Appendix 2.
Annual Pilot Budget Request Guide
CATEGORY OF EXPENSE |
ALLOWABLE REQUEST ON PILOT PROJECT? |
| Books, Subscriptions | No |
| Computers, Laptops | No |
| Consultative Services | No |
| Equipment | No |
| Expenses in Obtaining a Visa | No |
| Graphics, Photography Charges | No |
| Indirect Costs | Will be added by the CTSA administrative office in accordance with negotiated F&A rate agreement |
| Lab Tests - Clinical | Yes, justify and verify the costs with the laboratory |
| Lab Tests - Research - Core Services | Yes, justify and verify the costs with the laboratory |
| Malpractice Insurance | No |
| Membership Dues | No |
| Office Supplies | No |
| Parking Fees | No |
| Personnel Recruitment | No |
| Personnel: | |
| -Principal Investigator / Co-investigator Salary / Fringes | Maximum of $5,000 in salary support for established investigators who do not currently have active NIH support as a PI or co-PI; established investigators with active NIH support as PI or co-PI are ineligible for salary support. There is no salary limit for early-stage investigators (early-stage is defined as "has not been a PI for a substantial NIH research award"). |
| -External Employee | No |
| -Technical Support Personnel (study coordinator, lab tech, nurse, procedure tech, student) | Yes, up to $20,000/year (before fringe benefit costs) to support research assistants or personnel |
| Publication Costs and Reprints | No |
| Receptions and Meals | No |
| Scientific Meeting Fees and Expenses | No |
| Service Contracts for Equipment Maintenance | No |
| Software Packages | Yes, if unavailable and essential to the project/strong justification required. |
| Space Alterations and Renovations | No |
| Stipend for Medical Students | Only if they are research personnel |
| Subject Participation Reimbursement | Yes |
| Lab Supplies, Disposables | Yes, provide detailed justification - must be relevant to the proposed research and must be "consumed" by the project. |
| Telephone Long Distance (related to project) | No |
| Travel - Domestic or Foreign | No |
| Tuition Costs | No |
| Uniforms, Wearing Apparel | No |
| ANY NON-LISTED ITEM OR CATEGORY | Please contact the CTSC office. |
Appendix 3.
Application Submission Process for Invited Full Proposals Due date February 14, 2025
The submission deadline is 11:59 PM on February 14, 2025. Only those applicants with approved LOI’s will be invited to submit a full application via the InfoReady Grant Management System.
Submissions must be made by the PI or on behalf of the PI through PI’s InfoReady account. Submissions made under anyone else’s name will not be accepted.
Completeness of Application
Complete applications will consist of:
- The online submission
- eRA commons username of the PI & SPARC Request ID (SRID)
- Short summary of the work directed to the lay public (500 characters)
- Dollar amounts of other support currently available to all investigators
- NIH Biosketches of the PI and all co-investigators
- Active IRB and/or IACUC approval letter (if applicable). If required, no exceptions.
- Letter(s) of reference from CTSC Core program director or his/her designee documenting the PI’s consultation with Core management if a CTSC Core Program is being utilized
- Detailed budget and budget justification. Details must include costs per unit x number of units needed and/or cost per hour and number of hours required. Applications lacking sufficient budgetary detail will be returned to the applicant.
- Research proposal (see details below)
- References and figures may be uploaded as a PDF or Word document in the Appendix section of the application.
- Letter of support from PI’s department to ensure the investigators have sufficient protected research time and facilities to conduct the proposed research.
- Failure to submit ALL documents before 11:59 PM EST of submission date constitutes an incomplete application. Incomplete applications will not be reviewed.
Research Proposal
The research proposal (maximum 5 pages based on Arial font size 11, 1⁄2” margins) must be uploaded as a PDF within the application. The research proposal will include:
- Background and significance
- Preliminary studies
- Description of the study hypothesis, design, expected results, expected timeline, and feasibility
- Relevance and benefit to the CTSC/Translational Research and the anticipated results and probability that this project will lead to applications for extramural funding.
- If the study requires IRB approval, include an IRB statement including plans to obtain IRB approval by April 30, 2025, dates of submission and/or approval. (An approved or nearly approved IRB will be favorably reviewed.)
- Has this project been submitted to or received Intellectual Property protection? If yes, do you have a plan to file an invention disclosure? If no, would you like help to learn more?
- Statement whether this project is planned to have any foreign components, MOUs, or sub-awards
F.A.I.R. Principles and Rigor & Reproducibility Standards
The research proposal must demonstrate the use of F.A.I.R. Principles and Rigor & Reproducibility Standards throughout the project. For more information and definitions, review the Nature article: The FAIR Guiding Principles for scientific data management and stewardship and NIH’s Policy and Compliance website: Enhancing Reproducibility through Rigor and Transparency.
Upload the Research Proposal into the application as a PDF document. The appendix is limited to 5 pages. No abstracts.
Funding decisions will be made on or about April 21, 2025. Any applications with IRBs must have IRB approval IN PLACE prior to April 30, 2025.
Review Process – Application
- All awards that will involve “Applicable Clinical Trials” are required to register on clinicaltrials.gov before enrollment of the first subject. For additional information about registering clinical trials visit: http://prsinfo.clinicaltrials.gov/fdaaa.html
- Recipients of the pilot awards must adhere to Federal, State, and local guidelines with respect to scientific conduct of research, conflict of interest policies, human subject participation, and use of animals, hazardous or radioactive materials, and recombinant DNA in their research studies.
- The CTSC Scientific Review Committee (SRC) will review proposals. When appropriate, external experts/reviewers will be asked to participate in the review process by the CTSC SRC.
- Reviewers will rate the proposal according to the NIH Scoring Scale and provide comments as appropriate to the Committee via InfoReady.
In making a decision, the SRC will take into consideration the following:
- Is this proposal likely to advance innovative, multi-center clinical studies that integrate community and clinical partners to ensure health improvements for all?
- Is this proposal likely to advance Dissemination & Implementation of research evidence and innovation to Clinical Practice?
- Is this proposal likely to advance Interventions at Community and/or Clinical Practices to promote Health Equity?
- Overall rating of this proposal.
- Significance and originality of the proposal.
- Feasibility, the ability to determine the answer to the premise within 12-months.
- Likelihood the outcome will lead to subsequent funding or success however defined by the proposal.
- Budget Justification
The review committee would like to emphasize the importance of (a) providing a plan for CTSC resource use or enhancement; (b) specifying plans for how the project will lead to funding from other federal and non-federal granting agencies.
Proposals will be reviewed and either approved or disapproved. All applicants receive feedback from blinded peer reviewers. Disapproved projects may be re-submitted to the next Annual Pilot Grant period.
Appendix 4.
Requirements from NIH-NCATS for the CTSC Pilot Program
IRB and IACUC Approvals: All IRB and IACUC protocols must be approved prior to expenditure of funds. Any applications with IRBs must have IRB approval IN PLACE prior to April 30, 2025.
Delayed Onset Human Subjects Research: The NIH requires that the CTSC obtain explicit approval from the NIH for any pilot-funded research involving human subjects. Accordingly, the IRB-approved protocol and other materials must be submitted to the NIH at least 45 days prior to the project start date. CTSC personnel will work with awardees to meet these requirements.
Prior Approval of Vertebrate Animals Research: The NIH requires that the CTSC obtain explicit approval from the NIH for any pilot-funded research involving vertebrate animals. IACUC approval documentation and other materials must be submitted to the NIH at least 45 days prior to the project start date. CTSC personnel will work with awardees to meet these requirements.
Use of Application Information
The CTSC will not distribute information about submitted proposals to anyone without the applicant’s permission except to the individuals assigned to review the application. However, the CTSC may ask the applicants for permission to use the title of their application and/or the lay summary for promotional purposes. Permission will be obtained in writing and applicants have the right to decline if they so choose. Please contact Rachael Massey at rachael.massey@uhhospitals.org with any questions you may have about this.
Service as a Reviewer
Awardees will be included in a list of researchers to serve as potential reviewers on future CTSC Pilot Grants. Depending upon your specialty and area of expertise, you may be contacted by the Pilot Program Director to review applications.
Appendix 5.
Reporting Process
Grantees are required to submit an interim report at 6 months and a final report no later than 60 days after the award end date that summarizes major activities and research findings. The CTSC Pilot Grant Program will also contact the awardees on an annual basis (or more frequently as NIH requirements dictate) to request information concerning the funding status of the research initiated with the CTSC award as well as related publications for a period of ten years after the end of the funding period or until the line of research has concluded. Awardees may be asked to present their findings at an annual CTSA retreat.
Publications
A copy of any manuscripts or abstracts accepted for publication/presentation, which contains any results found using funds from the CTSC should be sent to the CTSC Pilot Program Coordinator upon notification of acceptance.
Support from the CTSC MUST be acknowledged when findings are reported, published or publicity is given to the work. All pilot award recipients must agree in writing to cite the CTSC award on all publications resulting from funds provided from the CTSC to the investigator making it possible to publish. Please include the following text: “This project was supported by the Clinical and Translational Science Collaborative of Northern Ohio which is funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Science Award grant, UM1TR004528. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH." It is imperative to note that publications resulting from this award obtain a PMCID as mandated by the NIH’s public access policy.
Any current or past awardee that does not acknowledge publications and research as a result from this award will not be eligible for future funding or support from the CTSC.
Public Access Policy Reminder – The Director of the National Institutes of Health shall require that all investigators funded by the NIH submit or have submitted for them to the National Library of Medicine’s PubMed Central an electronic version of their final, peer-reviewed manuscripts upon acceptance for publication, to be made publicly available no later than 12 months after the official date of publication: Provided, That the NIH shall implement the public access policy in a manner consistent with copyright law.
Patents
Awards are made with the understanding that the CTSC will receive written notification of the filing of a patent application for any discovery made based on work funded by these awards.
Data Sharing
In accord with NIH policy, all primary research data generated with CTSC support will be available for sharing no later than the acceptance for publication of the main findings from the final data set. Even if primary research data are stripped of all personal identifiers, it is possible for deductive disclosure of subjects with unusual characteristics. Therefore, in order to maintain privacy (per HIPAA), data and associated documentation will be available only under a data-sharing agreement that provides for: 1. a commitment to using the data only for research purposes and to NOT identifying any individual participant; 2. a commitment to securing the data using appropriate computer technology; and 3. a commitment to destroying or returning all data after analyses are complete. The data sharing agreement will also require acknowledgement of the CTSC as the source of data and will request pre-release review of any presentations or publications by the CTSC PI (or the PI who generated the primary data). Agreement to provide financial support for itemized specific expenses of data sharing may also be required in the data sharing agreement.
Next steps
- Read through the RFA thoroughly
- Register for the Annual Pilot Program Technical Webinar
- Schedule a consultation through SPARC with the CTSC Pilot Program team
- Develop and submit your Letter of Intent (LOI)
- Two step process!!
- Create Project ID in SPARC
- Submit LOI in InfoReady
- Two step process!!
Reach out to Anna (abt11@case.edu) with any questions!
FAQs
Applying for the Annual Pilot Award Program is a two-step process:
-
You will first need an ID number for your project from SPARCRequest (called a "Project ID"). Visit SPARCRequest and add "Annual Pilot" under Pilot Funding Opportunities to your cart and continue through the prompts to complete your submission.
-
Once you have obtained your "Project ID", continue to InfoReady to submit your application for the applicable funding opportunity.
How do I find my SPARCRequest ID (SRID)?
- Log into SPARCRequest with your CWRU Single-Sign On ID and password
- In the search bar, search “annual pilot”
- Click on “Service: Annual Pilot”, then click the green box “Yes”
- Follow the prompts to submit your request
- At the end of the prompts, you will be given a SRID (SPARCRequest ID)
For additional information about the SPARCRequest ID, please see the CTSC's website.